





 ver the years, interest in ¹H MRS increased rapidly, and it is now more common than ³¹P MR spectroscopy. Other nuclei like ¹³C and ¹⁹F are becoming more readily accessible by standard equipment.
ver the years, interest in ¹H MRS increased rapidly, and it is now more common than ³¹P MR spectroscopy. Other nuclei like ¹³C and ¹⁹F are becoming more readily accessible by standard equipment.
Table 05-02 shows some important properties of selected nuclei which are of interest in biological studies.
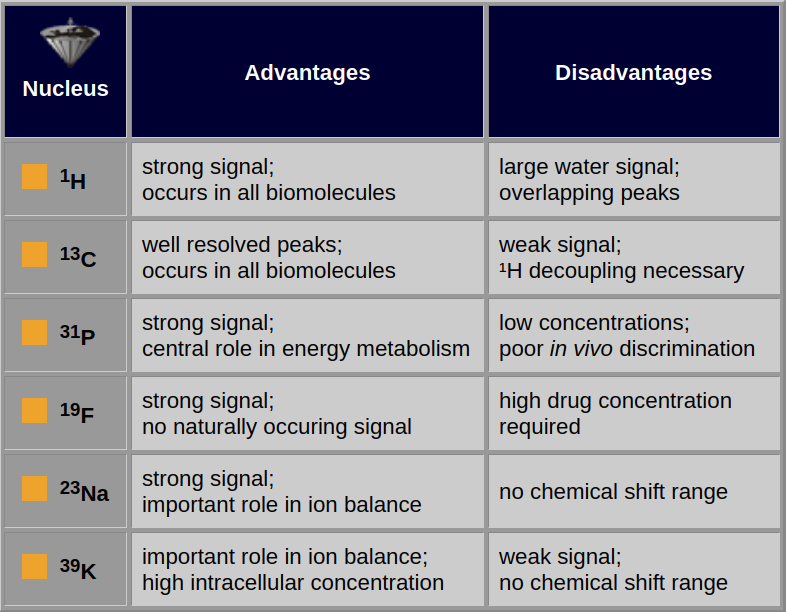
Table 05-02:
Advantages and disadvantages of selected nuclei for MR spectroscopy.
The spin quantum number, n, is a fundamental property of the atomic nucleus. Among other things, it is known that the nuclear spins can occupy 2n+1 energy levels, so nuclei with a spin of 1/2 have two possible energy states, whereas nuclei with a spin of 3/2 have 4 possibilities.
Nuclei with spins larger than 1/2 are said to be quadrupolar. An important practical feature of quadrupolar nuclei is that their relaxation is sensitive to fluctuating electric fields, as well as fluctuating magnetic fields, so that their T1 and T2 times are much shorter than for nuclei with a spin of 1/2.
Since one wants as much signal as possible, it is desirable for a nucleus to have a high sensitivity, although the natural abundance is also important.
The relative sensitivities of ³¹P and ¹³C differ by about a factor of 4, but because ³¹P is 100% abundant and ¹³C is only 1.1% abundant (i.e., about 98.9% of carbon nuclei are the nonmagnetic ¹²C isotope), the absolute sensitivities differ by about 400 (Table 05-03).
Alternatively, ³⁹K nuclei are about 31 times less sensitive than ¹³C nuclei, but because the potassium isotope is 93% abundant and the carbon isotope is 1.1% abundant, a sample containing potassium would produce a stronger signal than a carbon sample at a similar concentration.
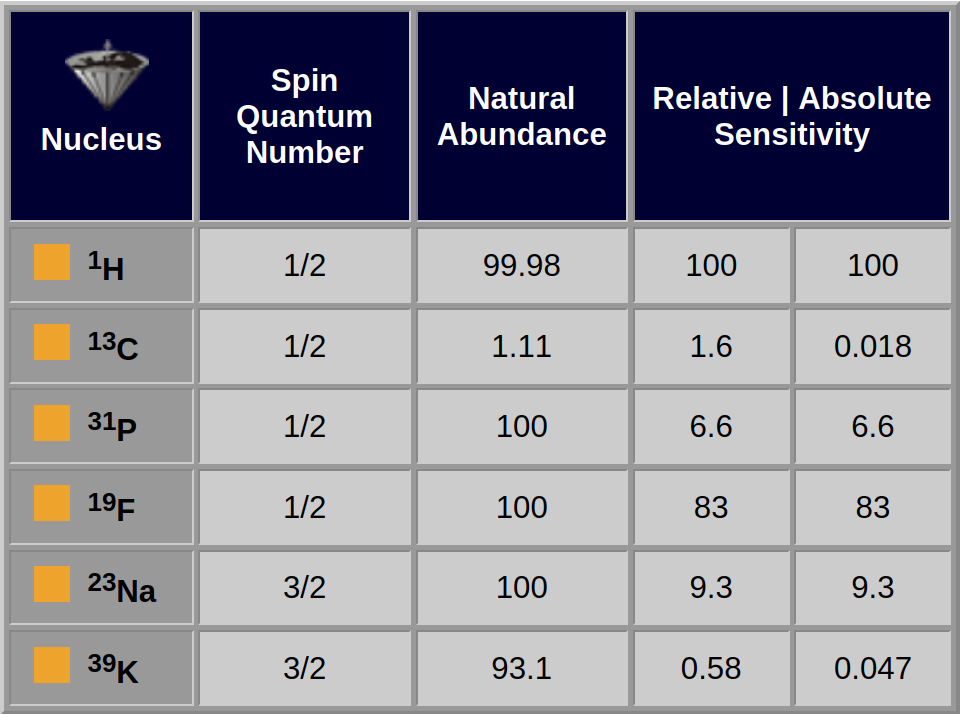
Table 05-03:
Important NMR properties of selected nuclei used for in vivo MR spectroscopy.
¹H studies have become increasingly popular as technical difficulties have been overcome and as interest has switched to areas of metabolism which lack phosphorylated metabolites (Figure 05-06). ¹H possesses the strongest response of all the atomic nuclei, and it is found in all biochemicals. Thus, it is a good nucleus for monitoring metabolism [⇒ Gadian 1990, ⇒ Matson 1999, ⇒ Miller 1991].
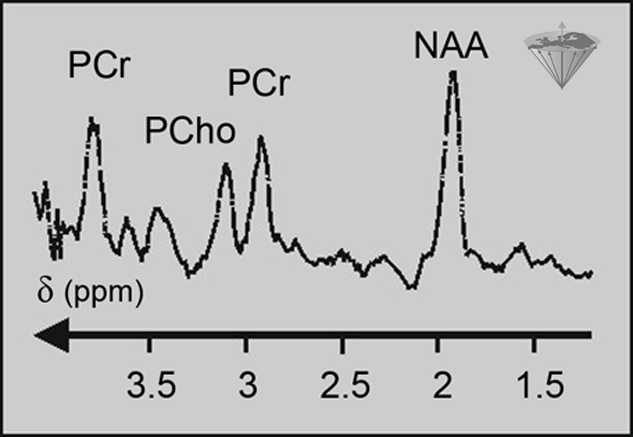
Figure 05-06:
Proton spectrum of a normal human brain. PCr: (phospho) creatine; PCho: (phospho) choline; NAA: N-acetylaspartate.
However, there are some major technical problems. Probably the biggest problem with ¹H MRS is the large signal from water in tissue. If we assume a rather conservative figure for tissue water content of about 65%, then the molar concentration of the water would be about 36 M. Since there are two H nuclei in each molecule of water, this gives a ¹H concentration of over 70 M.
The metabolites which we wish to observe have a maximum concentration of 10 mM or less, which is at least 7,000 times smaller than the water signal. Therefore, special methods are required for reducing the size of the water signal to a level where it is comparable to that of the metabolites. The simplest method is to use a long selective frequency saturating pulse, but whereas this is very effective in vitro, it can lead to unacceptable heating of tissue in vivo.
Multiple pulse sequences, such as the binomial pulse sequences, can be used to reduce the signal from water. If the ¹H nuclei in water are not excited, then they cannot give rise to a signal.
Another method of water suppression exploits the characteristics of a T1 relaxation curve. A selective 180° pulse is used to invert the water magnetization. At first the magnetization will be large and negative, but relaxation processes will start to take it back to its equilibrium values. After 0.69×T1 of water, the water magnetization will be approximately zero. At this point a 90° excitation pulse would produce a relatively strong signal from the metabolites and very little signal from the water.
Such methods are also applied in MR imaging (cf. Chapter 11).
If we were using a spin-echo pulse sequence in association with a particular volume selection method then we could arrange for the echo to be formed at the appropriate time after the selective inversion pulse so as to produce a minimum water signal.
Another problem with ¹H MRS is the narrow dispersion of the ¹H peaks. They lie in quite a narrow frequency range, thus there is a lot of overlapping. The problem can be helped by working at higher magnetic field strengths, but although ultrahigh-field whole-body systems do exist, the majority of MR spectroscopy is performed at 1.5 and 3.0 Tesla on equipment for which MR imaging rather than MR spectroscopy considerations dictate the field strength.
Clinical applications of proton MRS of the human brain include epilepsy, space-occupying lesions, multiple sclerosis, degenerative diseases, among them Alzheimer’s, Parkinson’s, and Huntington’s, hypoxia, and some metabolic diseases. A fine overview and review of clinical ¹H MRS in central nervous system disorders was published by Öz and numerous co-authors from different centers in 2014 [⇒ Öz 2014]. There are also other diseases which have been studied using proton MR spectroscopy, particularly diseases of the muscular system.
Unlike ¹H and ³¹P, the magnetically active isotope of carbon, ¹³C, is not the most abundant form of the nucleus. ¹³C can be found in all biochemicals and the signals have a wide dispersion, i.e., they occur over quite a large frequency range, which reduces the likelihood of overlapping peaks.
The biggest drawback of ¹³C is the exceedingly weak signal and problems with ¹³C-¹H coupling. In a ¹H-coupled ¹³C spectrum, most of the peaks will be split into two or more smaller peaks, which complicate the spectrum and reduces the signal-to-noise ratio, but the coupling effect can be removed by decoupling techniques. Direct irradiation at the proton resonance frequency is the simplest option, although this can lead to heating of tissue in vivo.
Multiple pulse techniques are available which are just as effective as direct irradiation but use a fraction of the power. The need for decoupling means that for ¹³C MR spectroscopy, the instrument must be capable of operating two channels simultaneously, which increases complexity and also cost.
 One advantage of ¹³C MR spectroscopy is that we can perform labeling studies. By giving carbon-labeled compounds to an animal or patient, we can follow the large and distinctive signals from the labeled compound and try to work out how it is metabolized in the body. Because each carbon position in a molecule will produce a characteristic signal, the labeling experiment can be used to follow not only which molecules end up with the label but also the exact position in which they end up.
One advantage of ¹³C MR spectroscopy is that we can perform labeling studies. By giving carbon-labeled compounds to an animal or patient, we can follow the large and distinctive signals from the labeled compound and try to work out how it is metabolized in the body. Because each carbon position in a molecule will produce a characteristic signal, the labeling experiment can be used to follow not only which molecules end up with the label but also the exact position in which they end up.
This information is extremely useful in working out which biochemical pathways were used in the conversion of one molecule to another. Similar labeling studies are not possible with ³¹P and ¹H because they are already 100% abundant. Unfortunately, ¹³C MR spectroscopy labeling is fairly costly.
¹³C MRS can detect signals from sugars, lipids, and glycogen in the liver and in muscle. It can obtain information about the carbon balance of energy metabolism, which is complementary to the information obtainable by ³¹P MR spectroscopy about energy metabolism [⇒ Matson 1999, ⇒ Shulman 1990].
A promising application for ¹³C spectroscopy is the analysis of body fluids such as blood and urine. This can be done routinely with very high field analytical NMR spectrometers. For the same purpose, proton spectroscopy can be used.
¹⁹F has a strong NMR signal and is 100% abundant. Fluorine studies have been performed on the metabolism of fluorine-containing drugs; since there are no naturally occurring fluorine signals in the body, all fluorine signals must come from the drug or its metabolites.
The drawback of fluorine MRS is that despite the strong signal, we still need drug concentrations in the order of 1-10 mM in the tissue, which is a rather high concentration for many drugs.
The resonance frequency of fluorine is quite close to that of ¹H at the same magnetic field, and it is often possible to perform fluorine studies on the ¹H channel of MRS equipment without the need for major modifications.
²³Na and ³⁹K differ from the other nuclei mentioned because they do not have spins of 1/2. They both have a spin of 3/2 and are therefore quadrupolar nuclei. Both are high-abundance isotopes (²³Na is 100% abundant and ³⁹K is 93.1% abundant).
²³Na has quite high extracellular concentrations, whereas ³⁹K has quite high intracellular concentrations, and both have an important role to play in ion balance. A big difference between the two is that sodium has a reasonably strong signal, comparable to phosphorous, whereas potassium has a weak signal. The absolute sensitivity of ³⁹K is about three times that of ¹³C, but the potassium signal is much broader than the carbon signal because the T2 of potassium is very short. This is due to the effect of quadrupolar relaxation, which reduces the signal-to-noise ratio of the peaks.
³⁹K also has a very low resonance frequency, which adds to the technical difficulties of the experiment. Animal studies with potassium have been performed at 4.7 Tesla, but, to our knowledge, the nucleus has not been studied at 1.5 Tesla. Although sodium also has a broad signal due to quadrupolar effects, the much greater signal strength means that reasonable spectra can be obtained.
Unfortunately, ²³Na and ³⁹K have no natural chemical-shift dispersion. In other words, the entire signal from an in vivo sample comes at the same frequency. Methods do exist for separating the chemical shift of intracellular ²³Na and ³⁹K from extracellular sodium and potassium using chemical-shift reagents (rather like the relaxation contrast agents used in imaging), but studies are currently restricted to cells and animals [⇒ Kohler 1991, ⇒ Matson 1999, ⇒ Rashid 1991].