





 oth TOF-MRA and PC-MRA have limitations. Enhancement of blood can be erratic, mostly due to the influence of flow irregularities. In some body regions, motion of the surrounding organs by breathing, peristalsis or pulsation affects angiographic depiction of vessels negatively, and saturation effects influence signal intensity and contrast of blood vessels.
oth TOF-MRA and PC-MRA have limitations. Enhancement of blood can be erratic, mostly due to the influence of flow irregularities. In some body regions, motion of the surrounding organs by breathing, peristalsis or pulsation affects angiographic depiction of vessels negatively, and saturation effects influence signal intensity and contrast of blood vessels.
There are numerous other inherent MR properties which can easily deteriorate both TOF or PC images (Table 14-02). Thus, the dream of finally having a completely noninvasive imaging method was shattered once again. If MR angiography was to compete with x-ray angiographic methods, higher spatial and temporal resolution and more reliable enhancement would be necessary — with the application of contrast agents.
There are four different categories of possible angiographic agents. Figure 14-18 classifies them [⇒ Port 1999]:
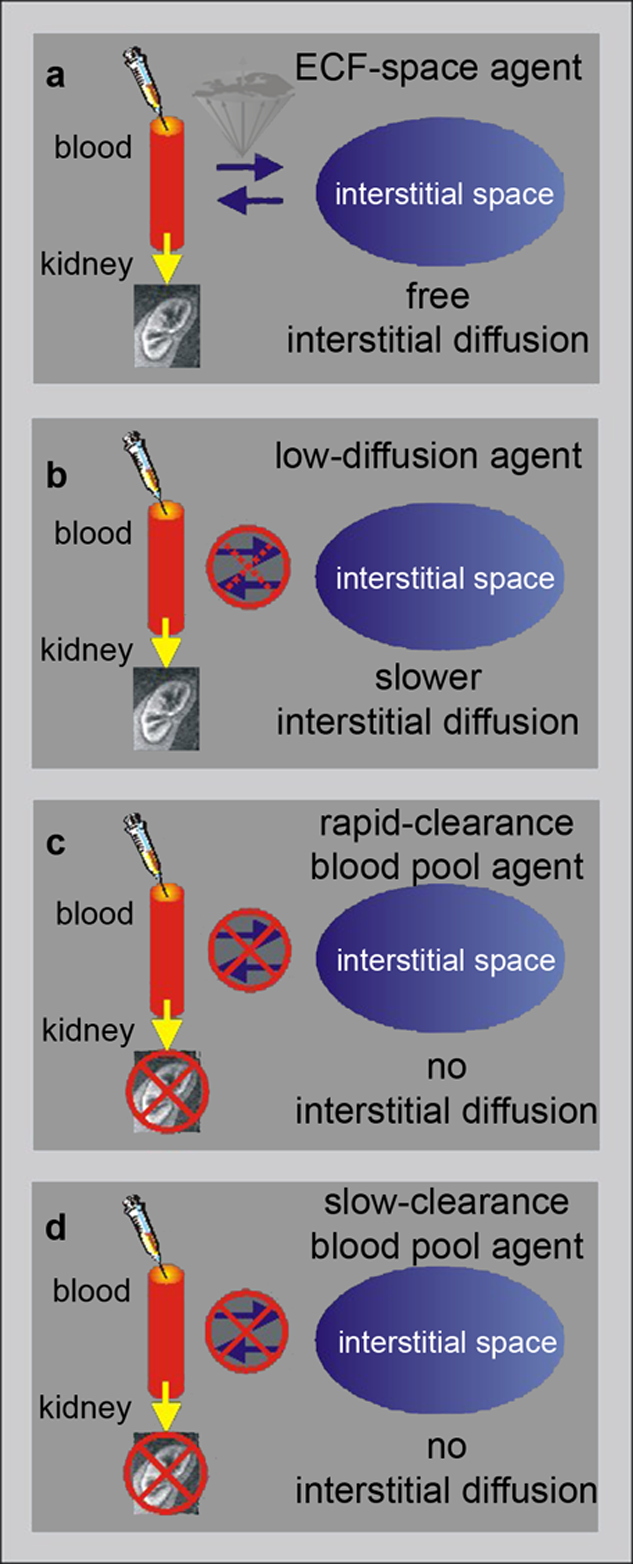
Figure 14-18:
Angiographic contrast agents (modified from Port).
(a) ECF-space agents; (b) low-diffusion agents; (c) rapid-clearance blood-pool agents; (d) low-clearance blood-pool agents.
Their categories are based on their global ability to cross the endothelium and to filter through the renal glomeruli.
Low-diffusion agents have an intermediate position between ECF-space and blood-pool agents, and their interstitial diffusion occurs at a lower rate than that of ECF-space agents.
Rapid-clearance blood-pool agents are mainly confined to the vascular space, but are freely excreted by the kidneys, whereas the renal excretion of slow clearance blood-pool agents is very restricted.
These agents remain in the blood for a significantly longer time and their tissue uptake is limited. However, their imaging window is wider; examination can even be repeated if necessary, although repeat examination are not recommended because of possible side effects of the contrast agents.
The ideal contrast agent for bright blood MRA would have a high r1 relaxivity to make T1 as short as possible and, to avoid spin dephasing effects, a low r2 relaxivity to keep: T2* > 2×TE.
 Besides gadolinium-based agents, ultrasmall superparamagnetic iron-oxide particles also seem to be well suited for MR angiography, with efficient and long lasting positive intravascular signal enhancement. These compounds remain almost exclusively in the intravascular space and selectively display the blood vessels.
Besides gadolinium-based agents, ultrasmall superparamagnetic iron-oxide particles also seem to be well suited for MR angiography, with efficient and long lasting positive intravascular signal enhancement. These compounds remain almost exclusively in the intravascular space and selectively display the blood vessels.
Due to their prolonged plasma half-life, they could also be used to enhance areas with vessels of varying permeability, and thereby reveal a certain tumor affinity. They also can help to define ischemia and reperfusion after treatment, for example of cerebral or myocardial infarction.
With appropriate calculative algorithms, such agents can also be used to estimate tissue blood flow in myocardial and cerebral ischemia, pulmonary embolism, the vascularization of transplants, and perfusion of tumors.
First and foremost for commercial reasons, mainly ECF-space agents are used for contrast-enhanced MR angiography which can provide excellent angiograms when combined with rapid T1-weighted GRE-imaging [⇒ Marchal 1991, 1992]. Blood-pool or iron-oxide are not available on the market (cf. Table 13-03).
Contrast-enhanced MR imaging depends mainly on T1 effects, less on TOF- or PC-imaging techniques.
When during and immediately after injection blood has the shortest T1 of all tissues, it will show the brightest signal and thus the blood vessels will be visible in the MIP image.
Furthermore, even in periods of slow flow (diastole for most vessels), there still is good signal from the blood which reduces ghosting artifacts and/or eliminates the need for cardiac synchronization. This makes CE-MRA much easier to perform.
After the slow injection of an ECF-space agent, its concentration in the blood will rapidly decrease. Depending on the type, only 50% of the dose remains in the blood after 5-10 minutes.
However, with bolus injections (injection time less than 60 seconds), the initial first-pass concentration is high; it decreases rapidly immediately after the end of the injection (cf. Chapter 16).
The contrast agent is diluted with the total blood-pool volume, it leaks from the capillaries into the extracellular space in many tissues (e.g., muscle), and it is excreted by the kidneys. Thus, for vascular imaging, these contrast agents are best used for imaging the first pass of the applied bolus.
 However, even with contrast agents, the scan time can still be relatively long (20 seconds to 2 minutes).
However, even with contrast agents, the scan time can still be relatively long (20 seconds to 2 minutes).
Therefore, it is necessary to keep the arterial concentration high continuously by injecting during the entire scan. As a rule of thumb, the duration of the injection is equal to or slightly shorter than the scan time (Figure 14-19).
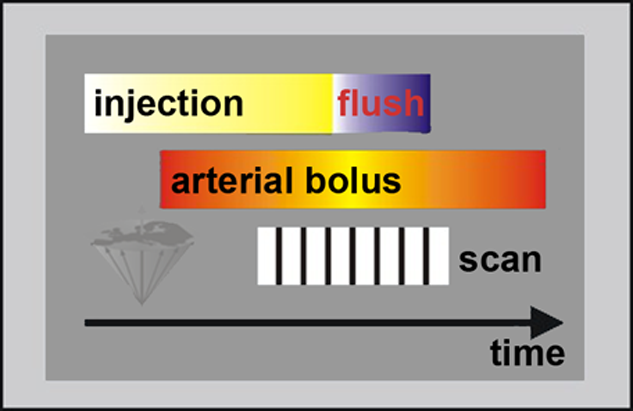
Figure 14-19:
Schematic drawing of a bolus injection for CE-MRA.
The delay between the start of the injection and the start of the scan depends on the delay between the start of the intravenous injection and arrival of the bolus in the arteries of interest.
The distance of the arteries of interest to the heart, the cardiac output, and the quality of the veins in which the agent is injected must also be taken into account (Figures 14-20 and 14-21).
The injected dose volume depends on the the contrast agent available and the allowed dose. Overdosed contrast agents applied for MR angiography have led to the NSF debacle.
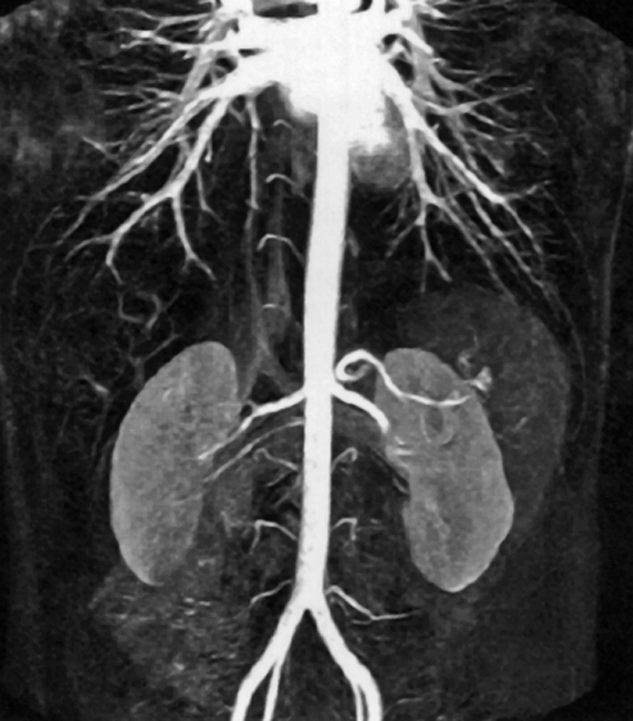
Figure 14-20:
CE-MRA of the abdominal aorta.
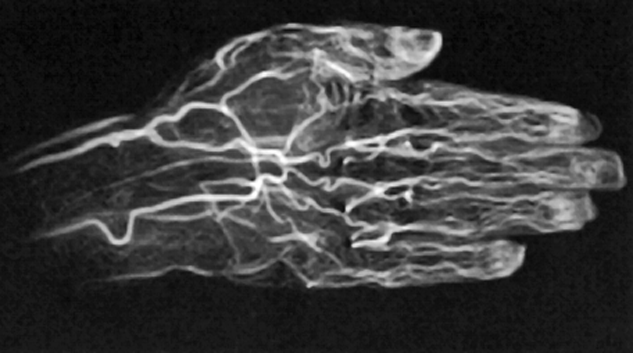
Figure 14-21:
CE TOF angiography of a hand.
In order to make sure that the arterial bolus is at its peak during imaging, several techniques can be used.
The delay between the start of the intravenous injection and arterial arrival of the bolus can be determined by a
The slice orientation of the test injection scan can be chosen in any direction, but if it is chosen perpendicular to the flow, presaturation slabs on both sides of the slice have to be used to suppress inflow effects so that only T1 effects will be visible.
Another approach is prospective bolus detection, where the acquisition is triggered by the arrival of the arterial bolus. Because of the time needed for breath hold instructions and the unknown delay between injection and arterial bolus arrival, both prospective and retrospective bolus detection can be problematic when combined with breath hold.
The protocol used for strong T1- weighting is relatively simple and is similar to that used in 3D inflow. The main difference is the flip angle and the freedom of slice orientation. Usually, a 3D gradient-echo sequence is applied.
To suppress background tissues, a short TR (typically between 5 and 15 ms, depending on gradient system and sequence) and a large flip angle (between 40° and 70°) are used.
Such a large flip angle cannot be used for a normal 3D inflow protocol (without contrast agent) because blood will become saturated too fast.
In combination with mechanical devices the entire peripheral vascular system can thus be examined after a single contrast agent injection (Figure 14-22). The combination of rapid automatic table movement and automatic injection and follow-up of the bolus allows multiple successive acquisitions.
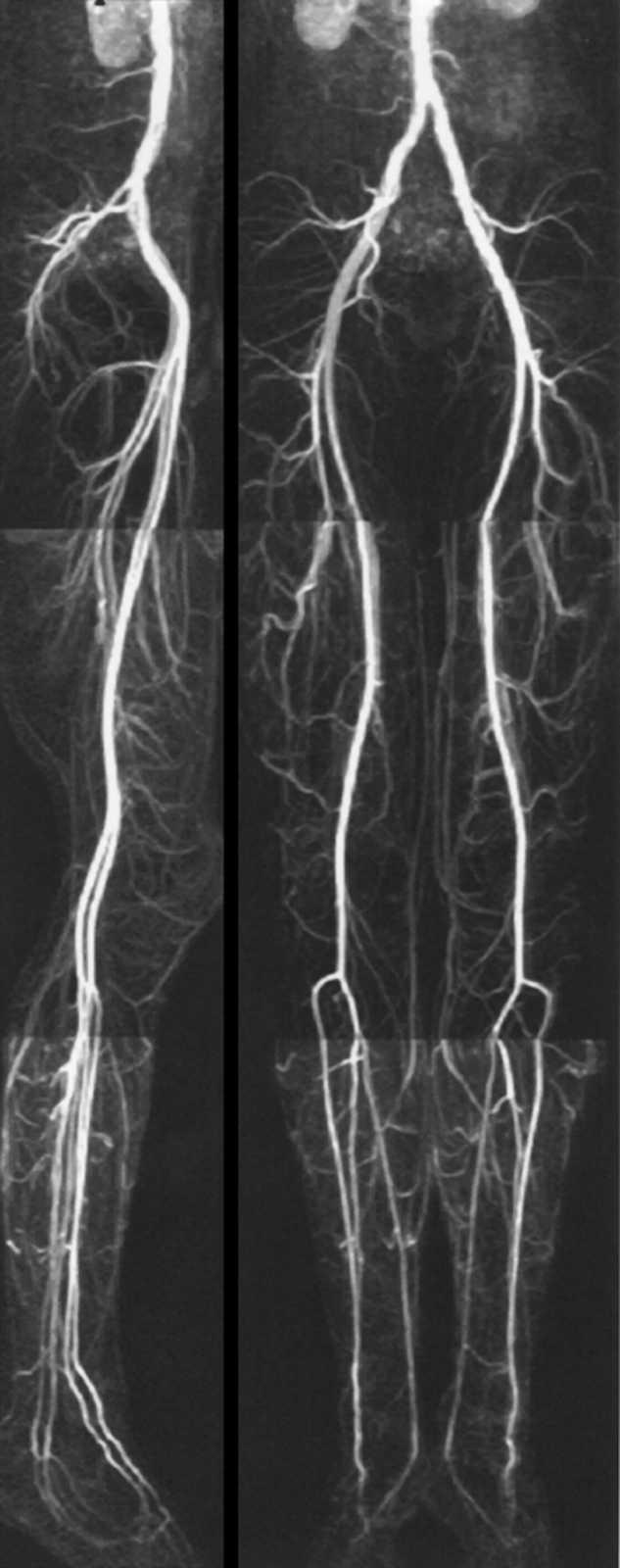
Figure 14-22:
Moving-bed CE-MRA of the pelvis and legs.
 The differentiation between arteries and veins is still problematic. The easiest differentiation is by morphology or, if a contrast agent is injected, by following the first pass.
The differentiation between arteries and veins is still problematic. The easiest differentiation is by morphology or, if a contrast agent is injected, by following the first pass.
Different approaches have been applied to distinguish between arteries and veins, both during image acquisition and by postprocessing image data. None of these approaches has been found to be sufficiently reliable. Among them is the presaturation method described in Chapter 17.
However, if presaturation slabs are used for selective demonstration of veins, the venous signal intensity in retrograde pathways may inadvertently be suppressed.
The use of gadolinium-based contrast agents obviates the dependence on inflow and allows imaging with large fields-of-view in the coronal or sagittal plane, despite substantial in-plane venous flow. Subtraction techniques offer a selective demonstration of veins, but a vein-free arterial study must be obtained first [⇒ Shinde 1999].