





![]() ow molecular weight paramagnetic contrast agents distribute into the intravascular and extracellular fluid (ECF) space of the body (see also: tissue uptake). Thus, they are also known as ECF space agents. Among the positive contrast agents, they are the most commonly and, basically, the only ones sold and used (Table 13-03). They are water-soluble and not tissue-specific. Their majority does not bind to protein.
ow molecular weight paramagnetic contrast agents distribute into the intravascular and extracellular fluid (ECF) space of the body (see also: tissue uptake). Thus, they are also known as ECF space agents. Among the positive contrast agents, they are the most commonly and, basically, the only ones sold and used (Table 13-03). They are water-soluble and not tissue-specific. Their majority does not bind to protein.
Their effect is caused by the metal ion in their center which contains unpaired electrons, and their relaxation depends on dose and magnetic field strength.
With its seven unpaired electrons and relatively long electron-spin relaxation time, gadolinium possesses the highest ability to alter the relaxation times of adjacent protons.
Because the metals suitable as relaxation agents and their salts are rather toxic, they have to be bound in stable complexes in which they are safely kept until the contrast agent is excreted. Such complexing organic molecules are called chelators, after the Greek word for claw. The bond should be as tight as possible to counteract the lability of the compound and to prevent the release of “free” gadolinium into the human body [⇒ Laurent 2001 + 2006]. Chelates come as linear (or stretched), or cyclic (macrocyclic) molecules (Figure 13-05 and Table 13-03).
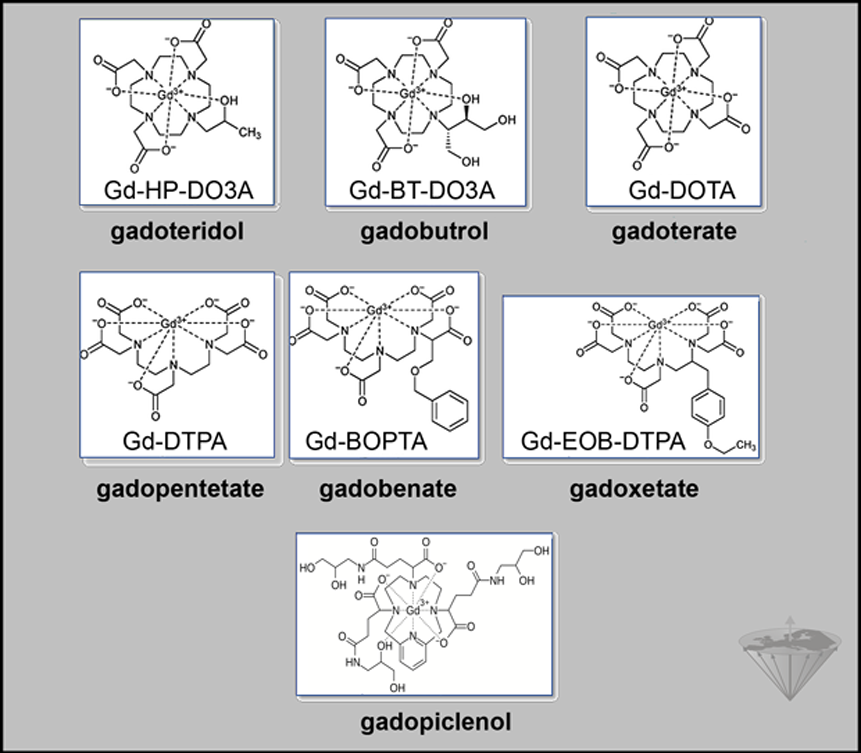
Figure 13-05:
Some Gd-based contrast agents (GBCA) on the market or withdrawn.
Among these chelators are DTPA, DTPA-BMA, DTPA-BMEA, DOTA, DO3A-butrol, and HP-DO3A. Bound to them, gadolinium forms low-molecular-weight water-soluble complexes (the final contrast agents) which are excreted through the kidneys. Gd-DTPA and Gd-DOTA are called ionic agents, whereas Gd-DTPA- BMA, Gd-DTPA-BMEA, Gd-DO3A-butrol, and Gd-HP-DO3A are called nonionic. In this context, the respective terms globally charged and globally neutral would be better. The relaxivities of all these agents brought to the market in the 1980s and 1990s are similar in water, but can differ in serum and plasma.
Further compounds include gadolinium bound to the chelators BOPTA, EOB-DTPA, and similar ligands that are slightly more lipophilic and excreted both via the kidneys as well as via the liver. The relaxivities of such agents can be higher because they may bind to protein. They are, however, extremely dependent upon field strength with a high peak around 0.7 T and, in the case of EOB-DTPA, a sharp drop afterwards, losing 30% of its relaxivity at 1.5 T and 60% at 3.0 T.
 Second generation agents. It has taken more than 30 years after the introduction of the first Gd-based MR contrast agent to introduce compounds with very high relaxavity so that the applied dose can be reduced. The first one is gadopiclenol (Elucirem, Vueway). It shows no protein binding and high kinetic inertness ( ⇒ Robic 2019, ⇒ Hao 2023, ⇒ Gendron 2024).
Second generation agents. It has taken more than 30 years after the introduction of the first Gd-based MR contrast agent to introduce compounds with very high relaxavity so that the applied dose can be reduced. The first one is gadopiclenol (Elucirem, Vueway). It shows no protein binding and high kinetic inertness ( ⇒ Robic 2019, ⇒ Hao 2023, ⇒ Gendron 2024).
Another one might be gadoquatrane ( ⇒ Lohrke 2022, ⇒ Hofmann 2024).
Figure 13-06 depicts the relaxivity behavior of some ECF-space agents versus field strength.
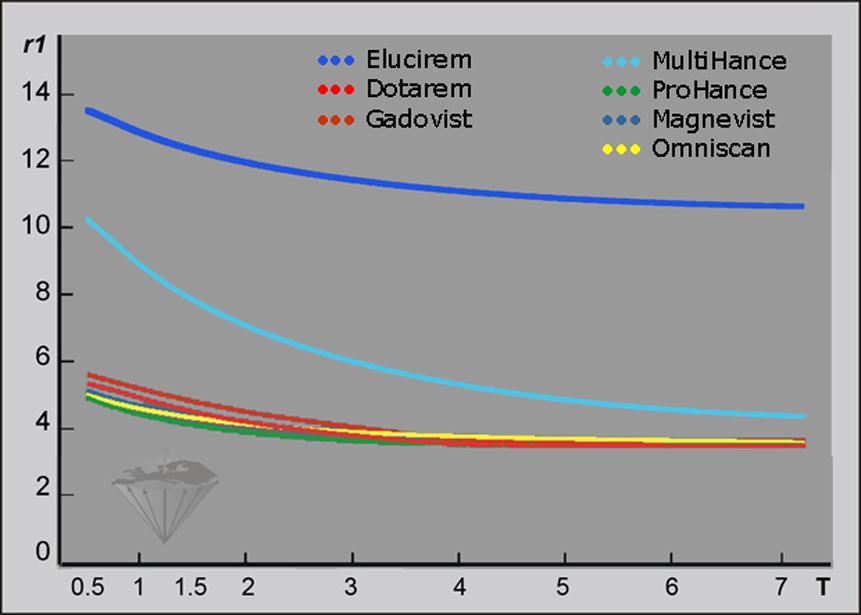
Figure 13-06:
Relaxivities r1 (in plasma; concentration 1 mM; per second; 37°C) of seven gadolinium based ECF-space contrast agents versus field strength (T = Tesla).
Exemplary r1 data at 1.5 Tesla (in plasma; standard deviation approximately 10%): Elucirem/Vueway 13.0, MultiHance 8.3, Dotarem 4.8, Gadovist 5.2, Magnevist 4.8, Omniscan 4.8, ProHance 4.5.
The relaxometric measurements leading to these data were performed at the NMR Laboratory of the University of Mons, Belgium.
The common clinical formulation of Gd-based ECF-space agents contains gadolinium at a concentration of 500 mM.
Increasing the dose of the agents above the recommended normal dose (i.e., higher than 0.1 mmol/kg body weight = 0.2 mL/kg body weight) may have both beneficial and unwanted effects (Figures 13-06 and 13-07). Depending on the field strength, it may facilitate the detection of small CNS lesions that have minimal blood-brain barrier breakdown.
As an exception, gadobutrol (Gadovist, Gadavist) is sold at twice the regular concentration (1M) and applied at half the volume of the other ECF-space agents (0.1 mL/kg body weight for 0.1 mmol/kg body weight). According to the US-American Food and Drug Administration, FDA, the unique molar strength of gadobutrol poses a risk for overdosage of the drug [⇒ Stinson]. However, since gadobutrol is a macrocyclic agent, giving twice the recommended volume (double the recommended dose) should not be considered harmful.
The recommended dose of gadopiclenol is only 0.05 mmol/kg body weight.
The intrathecal administration of gadolinium-based contrast agents for instance for MR cisternography might lead to serious neurotoxic complications such as death, coma, encephalopathy, and seizures [⇒ Patel 2020].
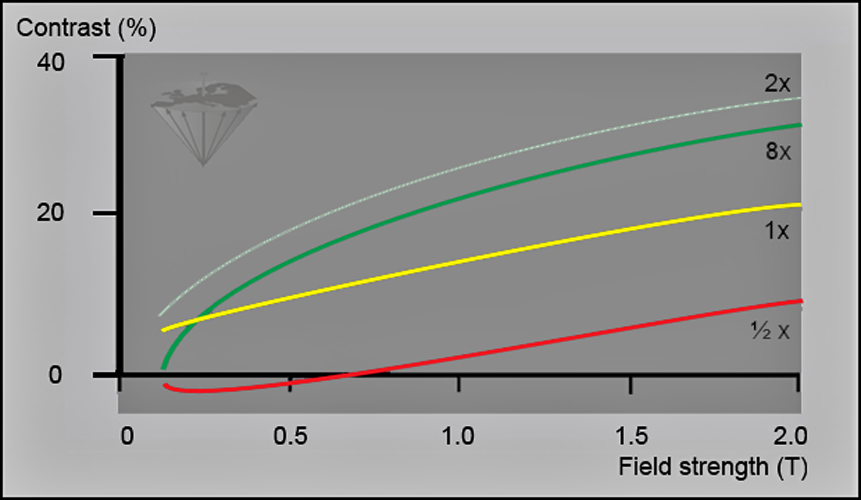
Figure 13-07:
Simulation of the influence of different tissue concentrations of Gd-based ECF space agents and magnetic field strength upon contrast in a glioblastoma. Strongly T1-weighted SE sequence, not field adjusted.
Red curve = half dose; yellow = regular dose of 0.1 mmol/kg body weight; light blue = double dose; green = octuple dose.
The curves in Figure 13-07 correspond to the contrast between a glioblastoma and white matter after enhancement. Before enhancement the contrast between glioblastoma and white matter is negative (the tumor is dark; its contrast behavior curve is below ½ dose but is not depicted in this figure) [⇒ Rinck 1999].
Only the regular (yellow) and double (light blue) doses give rise to sufficient contrast at all fields. Increasing them is counterproductive (8x). Figure 13-08 shows this behavior in a meningioma.
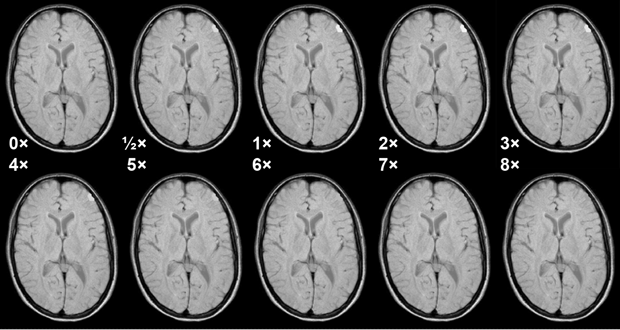
Figure 13-08:
Simulation of the influence of different doses of a Gd-based ECF-space agent in a meningioma at 1.5 Tesla. Medium T1-weighted SE sequence. Note that at higher doses the T1 enhancement effect of the agent vanishes because T2 takes over. Best enhancement is seen at the recommended dose and double the recommended dose.
Simulation software: MR Image Expert®

Figure 13-08-Video:
In this animation we see the same pictures as above and watch how the contrast changes.
Simulation software: MR Image Expert®
The figures explain too that in most cases cutting the contrast dose is also counterproductive.
Increasing dose, for instance in MR angiography, beyond the recommended dose or repeating contrast-enhanced examinations within a short time period may lead to late side effects (see: nephrogenic systemic fibrosis, NSF).
 Timing. The uptake of these agents is relatively fast. Conventional imaging exploits the uptake after 4-5 minutes; often image acquisition already starts after two minutes. Depending on the tissue, the local enhancement peak in the extracellular space is reached 5-30 minutes after injection.
Timing. The uptake of these agents is relatively fast. Conventional imaging exploits the uptake after 4-5 minutes; often image acquisition already starts after two minutes. Depending on the tissue, the local enhancement peak in the extracellular space is reached 5-30 minutes after injection.
Chapter 16 describes the organ and tissue transit of contrast agents after slow and after bolus injection in more detail.
 Imaging parameters. Because paramagnetic contrast agents are T1-agents, their effect is most pronounced on T1-weighted MR images, for instance on spin-echo images with short repetition and echo times, and gradient-echo images with short repetition times and high flip angle (50°-90°). Intermediately (ρ) weighted images will still show enhancement. The agents lose most of their efficiency on T2-weighted pictures; in many cases, even an isointense behavior can be observed (Figure 13-09).
Imaging parameters. Because paramagnetic contrast agents are T1-agents, their effect is most pronounced on T1-weighted MR images, for instance on spin-echo images with short repetition and echo times, and gradient-echo images with short repetition times and high flip angle (50°-90°). Intermediately (ρ) weighted images will still show enhancement. The agents lose most of their efficiency on T2-weighted pictures; in many cases, even an isointense behavior can be observed (Figure 13-09).
In general, imaging protocols should include precontrast T1-weighted images to exclude or differentiate high signal intensity pathologies such as hematoma, and T2-weighted images to exclude pathologies such as small edematous white-matter lesions. The acquisition of only pre- and postcontrast T1-weighted images cannot be recommended in brain studies trying to rule out unknown pathology.
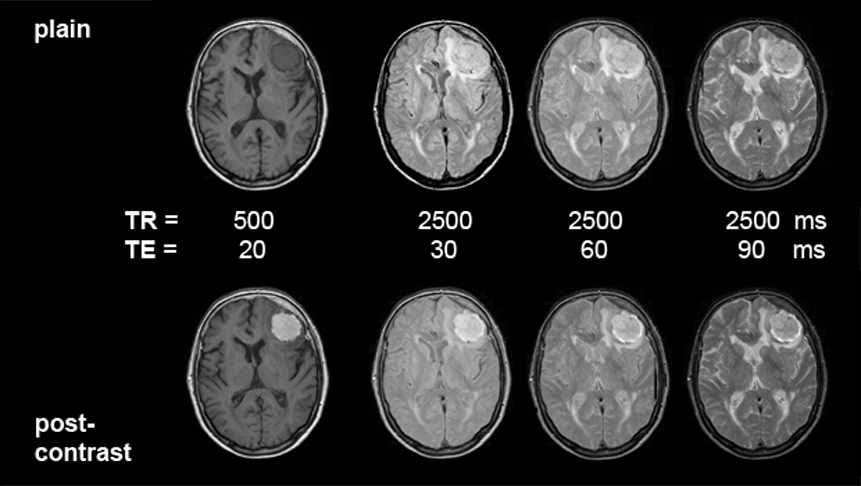
Figures 13-09:
Patient with a meningioma. Multiple SE sequence before (top row) and after (bottom row) intravenous application of a gadolinium contrast agent. The images to the left are heavily T1-weighted, then ρ-weighted, and to the right increasingly T2-weighted.
Simulation software: MR Image Expert®

Figure 13-09-Video:
Animation of contrast-enhanced images; patient with a meningioma; TR = 800 ms; TE between 20 and 120 ms (= T1-weighted to T2- influenced). The contrast-agent enhancement of the tumor slowly disappears (cf. Figure 13-08 a; plain images and enhanced images).
Simulation software: MR Image Expert®
The patient of the figure above has a huge meningioma in the left frontal lobe. It is easily visible on the non-enhanced images, mostly because of its mass effect and the bright surrounding edema on T2-weighted images. Yet, this case is a good example of the enhancement pattern of gadolinium contrast agents. This kind of tumor enhances brightly on T1-weighted images; there is still enhancement on ρ-weighted images. T2-weighted images, however, show the same contrast pattern before and after injection of the agent. If the meningioma or similar enhancing lesions are very small and no indirect signs of lesions can be found, only contrast enhancement will reveal the pathology (see also Figure 13-04 and Figure 13-08).
The diagnostic development of low molecular weight paramagnetic contrast agents was primarily focused on their use in lesions of the central nervous system (CNS), an indication in which plain MR imaging had found its first major field of application.
Stable ECF-space agents do not cross the intact blood-brain barrier (BBB). Thus, in the healthy CNS, clear enhancement is only seen in regions without this barrier, such as the choroid plexus. Normal enhancement in the brain is also seen in the pituitary gland and infundibulum, the cavernous sinus, dura mater, and nasal mucosa. Vessels may also enhance, particularly during the first pass of the contrast agent (Figure 13-10).
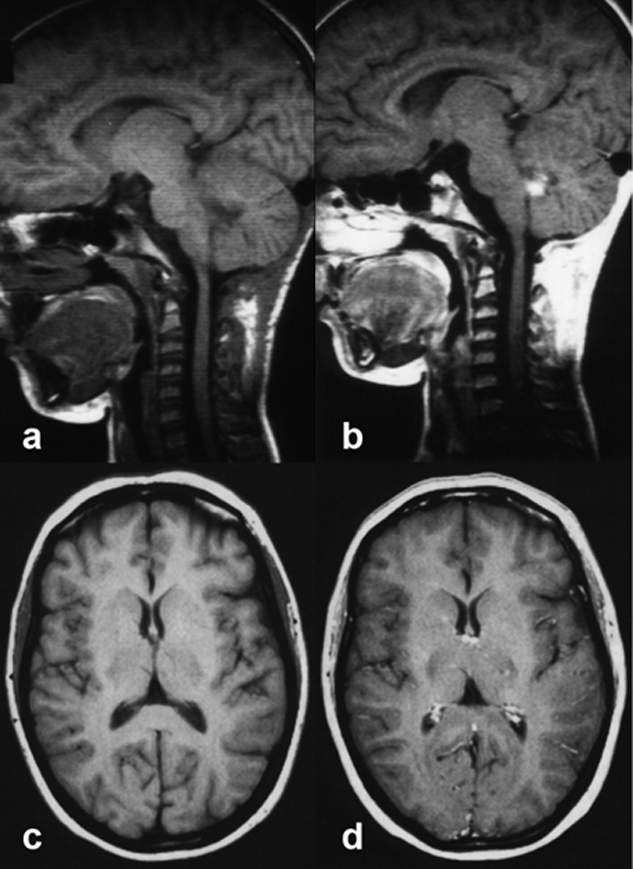
Figures 13-10:
Top: Child with a small ependymoma.
(a) before contrast application, (b) after contrast application.
The contrast agent enhances the tumor, but also other highly vascularized parts of the head, among them the pituitary gland and infundibulum, bone marrow, nasal mucosa, and blood vessels.
Bottom: Adult brain.
(c) before contrast application, (d) after contrast application.
Normal contrast enhancement in the choroid plexus and the superficial veins.
Pathological breakdown of the BBB allows paramagnetic contrast agents to cross straight into the extracellular space and to alter T1 relaxation locally. This occurs in a variety of pathologies such as tumors, infections, acute demyelination. The contrast enhancing effect of the contrast agent combined with the ease of demonstrating a lesion in different planes (sagittal, coronal, and transversal) with MR imaging, has proven to be of use in preoperative and preirradiation planning, as well as in follow-up during and after treatment.
Among other applications, Gd compounds have been found to be especially useful for increasing the detection rate of metastases and small tumors, and for improving tumor classification, the latter by allowing the differentiation of vital tumor tissue (well perfused versus impaired or absent blood-brain barrier) from central necrosis and from surrounding edema or macroscopically uninvolved tissue.
Isointense benign tumors like meningiomas and hamartomas are among the major indications for paramagnetic contrast agents. Acoustic neurinomas, in particular small ones within the internal auditory canal, are clearly enhanced.
In malignant tumors, contrast agents can help in delineating tumor and edema. Absence of enhancement is sometimes of as great a value as its presence, e.g., when distinguishing a low-grade astrocytoma at the cortical surface from a meningioma or small vascular white-matter lesions from metastases. Enhancement might also be seen in infections (e.g., toxoplasmosis and tuberculoma).
A more comprehensive coverage of clinical aspects can be found in relevant textbooks.
Figure 13-11 is an example of the ability of an intravenous Gd contrast agent to enhance brain tumors. The application of a contrast agent allows the delineation and definition of tumor extent — with certain limitations, as explained in the figure.
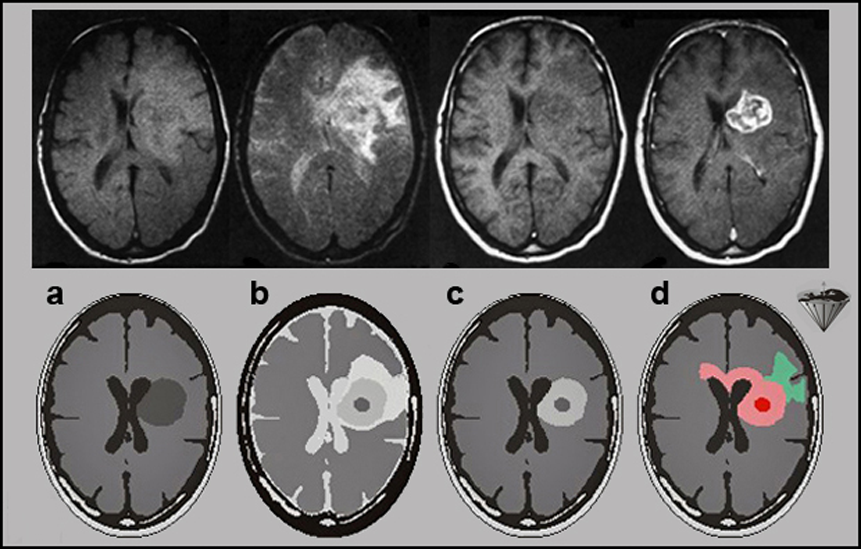
Figures 13-11:
Top row: Malignant brain tumor.
(a) proton-density-weighted image; (b) T2-weighted image; (c) T1-weighted precontrast image; (d) T1-weighted postcontrast image.
Although it is obvious that a large mass displaces the lateral ventricles, an exact delineation of the tumor is impossible on the first three images. Edema is well seen on the T2-weighted image, but the dark tumor areas are poorly delineated. Only after the contrast agent administration does the tumor become bright and its active parts are well visible.
Bottom row: Graphic explanation of corresponding histology. The contrast agent only enhances absence or breakdown of the blood-brain-barrier and high-vascularity lesions.
(a) and (b) T1- and T2-weighted precontrast images of a highly malignant tumor similar to the one seen in the top row; (c) the T1-weighted postcontrast image — corresponding to (d) in the top row; and (d) the actual microscopic tumor extension through the corpus callosum, which is not divulged by the MR images. The enhanced image shows only the tumor region, with the effect on the blood-brain barrier.
Applications outside the CNS include the musculo-skeletal system, ear-nose-throat diseases, the heart, kidneys and adrenals, gynecological diseases, lymphomas, joints, and the breast. For MR breast examinations application of a gadolinium based agent is mandatory [review article: ⇒ Rinck 1999b]; however, clinical studies revealed that in breasts with malignancy one of the compounds (gadobutrol, Gadovist) used for enhanced breast MR examinations overestimated the histologically confirmed extent of malignancy in up to 50 percent of the patients [⇒ GEMMA 2014].
One of the approaches to increase specificity was the exploitation of faster imaging techniques. These techniques reduced imaging times from several hundred seconds per image first to below 100 seconds, then to under 10 seconds. Now the uptake of contrast agents in different organs could be followed (dynamic imaging). Details of such techniques are described in Chapter 16.
 Acute adverse events and precautions. Gd-compounds are considered safe with acute adverse events happening in less than 1% of all patients; the number of side effects is at least one order of magnitude lower than adverse events with x-ray contrast media; this includes nephrogenic systemic fibrosis (see below). The most common side effects are headache, nausea and vomiting, as well as injection site reactions. In patients with a history of allergies and asthma the application of contrast agents should be carefully considered. Severe anaphylactoid reactions are rare, but have been reported. Proper supervision of the patient after injection, and access to an intensive care unit, must therefore be guaranteed [see, e.g., ⇒ Royal College of Radiologists 2015 and ⇒ American College of Radiology 2016].
Acute adverse events and precautions. Gd-compounds are considered safe with acute adverse events happening in less than 1% of all patients; the number of side effects is at least one order of magnitude lower than adverse events with x-ray contrast media; this includes nephrogenic systemic fibrosis (see below). The most common side effects are headache, nausea and vomiting, as well as injection site reactions. In patients with a history of allergies and asthma the application of contrast agents should be carefully considered. Severe anaphylactoid reactions are rare, but have been reported. Proper supervision of the patient after injection, and access to an intensive care unit, must therefore be guaranteed [see, e.g., ⇒ Royal College of Radiologists 2015 and ⇒ American College of Radiology 2016].
 Late adverse events (nephrogenic systemic fibrosis, NSF). In the early days of research and development of these contrast agents, their feared toxicity was associated with the possible competition with endogenous ions such as zinc and calcium. Such an exchange, called transmetalation, frees gadolinium from the chelate into the body. When dissolved in water, all commercially available complexes are very stable; only one molecule over several millions or billions releases its gadolinium.
Late adverse events (nephrogenic systemic fibrosis, NSF). In the early days of research and development of these contrast agents, their feared toxicity was associated with the possible competition with endogenous ions such as zinc and calcium. Such an exchange, called transmetalation, frees gadolinium from the chelate into the body. When dissolved in water, all commercially available complexes are very stable; only one molecule over several millions or billions releases its gadolinium.
When in the human body, however, challenged by other ions that want to replace gadolinium, these molecules behave differently. Macrocyclic compounds minimze this in vivo dissociation process. This instability was already pointed out in 1988 [⇒ Meyer, ⇒ Tweedle].
Gadolinium ions carry three positive charges. If the chelator brings three negative charges (as in Omniscan, ProHance, Gadovist, and Optimark), the global charge is zero (globally neutral). If it brings more (as in Dotarem, Magnevist, MultiHance, and Vasovist), the global charge is negative (globally charged). This is not the key to stability, however, since the macrocyclic compounds are the most stable regardless whether they are charged (gadoterate — Dotarem) or neutral (gadoteridol — ProHance and gadobutrol — Gadovist; cf. Figure 13-05). For the other contrast agents, the so called open-chain or linear chelates (Omniscan, Magnevist, MultiHance, Vasovist, and Optimark), the situation is different: analytical tests show that their ability to retain their gadolinium ion is weaker. Among these compounds, the negatively charged Magnevist, MultiHance, Vasovist, and Optimark are more stable than the neutral Omniscan [⇒ Laurent 2001, 2006, ⇒ Pietsch, ⇒ Sieber, ⇒ vander Elst].
Thus, from a chemical point of view, certain agents minimize the risk of late side effects and are to be preferred over others. In general, do not exceed the recommended dose and allow a sufficient period of time for elimination of the drug from the body prior to any readministration.
High-dose and/or repetitive examinations have led to severe (deadly or mutilating) late side effects (nephrogenic systemic fibrosis, NSF) in patients with severe renal failure and acute kidney injury. This was first described in 1997; the connection to gadolinium contrast agents was made by Grobner in 2006 [⇒ Grobner]. An "epidemic outbreak" happened in Denmark shortly later [⇒ Bennett, ⇒ Marckmann].
In general, where a specific agent was identified, the most commonly reported agent was gadodiamide (Omniscan), followed by gadopentetate dimeglumine (Magnevist), and gadoversetamide (Optimark) [⇒ Krefting].
Omniscan, Optimark, and Magnevist must not be used in patients with severe kidney problems, in patients around the time of liver transplantation, and in newborn babies less than four weeks of age, who are known to have immature kidneys; their dose should be restricted to the minimum recommended dose in patients with moderate kidney problems and infants up to one year of age, and there should be at least a period of seven days between scans; as a precaution, breastfeeding should be discontinued for at least 24 hours after the patient has received a high-risk agent. A precautionary suspension of breast-feeding following the administration of low-risk gadolinium-containing contrast agents is unnecessary. In general, breastfeeding should not be interrupted after gadolinium administration [⇒ American College of Obstetricians and Gynecologists 2017].
All patients should be screened for kidney problems using laboratory tests before receiving these agents [⇒ European Medicines Agency (EMEA) 2010].
Gadolinium deposits were also found in brain tissue after serial MR examinations in patients without kidney disease. There is strong evidence that the deposit of gadolinium can be traced back to the linear agents gadodiamide (Omniscan) and gadopentetate dimeglumine (Magnevist), but not to the macrocyclic gadoterate meglumine (Dotarem) [⇒ Kanda 2014 + 2015, ⇒ Radbruch]. In preliminary animal experiments it could be shown that the deposited gadolinium was partially cleared over 20 weeks with no detectable neurotoxicity [⇒ Smith 2017].
In general, for contrast-enhanced MRI examinations preference should be given to macrocyclic agents, as well as agents excreted by both the liver and the kidneys.
Risk categories according to the European Medicines Agency
(decision of the European Commission issued on 1 July 2010):
High risk: gadoversetamide (Optimark), gadodiamide (Omniscan) and gadopentetic acid (for instance: Magnevist, Magnegita, and Gado-MRT-ratiopharm);
Medium risk: gadofosveset (Vasovist), gadoxetic acid (Primovist), and gadobenic acid (MultiHance);
Low risk: gadoteric acid (Dotarem), gadoteridol (ProHance), and gadobutrol (Gadovist).
Finally, on 10 July 2017, the European Medicines Agency recommended the suspension of the marketing authorisations of gadoversetamide (Optimark), gadodiamide (Omniscan) and gadopentetic acid (Magnevist, et al.).
The FDA [⇒ US Food and Drug Administration] did not restrict any Gd-containing agents, stating: "To date, the only known adverse health effect related to gadolinium retention is a rare condition called nephrogenic systemic fibrosis (NSF) that occurs in a small subgroup of patients with pre-existing kidney failure … A review to date has not identified adverse health effects from gadolinium retained in the brain after the use of gadolinium-based contrast agents."
However, in 2018 FDA warned that gadolinium-based contrast agents are retained in the body.
The sad story of late side effects of a number of gadolinium-based contrast agents is another episode of ignorance and daredivilry at other people's (= patients') expense.
… and some years later: The background history how this could happen and how it happened.
Both x-ray and MR contrast agents can interfere with a number of blood tests. Such tests should not be performed for 48 hours after a contrast-enhanced x-ray or MR examination.