





 ne of the ultimate goals of contrast agent research in MR imaging is the development of compounds that actively identify specific tissues or pathologies. In order to target organs and to obtain higher disease specificity, iron oxides and liposomes have attracted particular interest. To optimize such agents, one has to evaluate at least three parameters:
ne of the ultimate goals of contrast agent research in MR imaging is the development of compounds that actively identify specific tissues or pathologies. In order to target organs and to obtain higher disease specificity, iron oxides and liposomes have attracted particular interest. To optimize such agents, one has to evaluate at least three parameters:
 Improvement of tolerance — although the tolerance of existing compounds is already very good; this includes chemical and biological inertness, as well as complete elimination from the body;
Improvement of tolerance — although the tolerance of existing compounds is already very good; this includes chemical and biological inertness, as well as complete elimination from the body;
 improvement of the enhancing effect; high and ultrahigh fields might require a different contrast agent than low and medium/high fields;
improvement of the enhancing effect; high and ultrahigh fields might require a different contrast agent than low and medium/high fields;
 selective distribution in the body to reach high local concentrations (organ or pathology specific tracers).
selective distribution in the body to reach high local concentrations (organ or pathology specific tracers).
Liposomes
Liposomes are one group of particulate contrast agents. Paramagnetic ions can either be encapsulated in the aqueous compartment of the liposomes or be linked to their lipid bilayers. Both manganese and gadolinium chelates have been attached to liposomes and studied preclinically. More sophisticated liposome compounds have been proposed, among them phospholipid spin-labelled and amphipathic chelate complexes. None of these compounds are clinically used.
Iron-based Contrast Agents
Iron chelates. In the 1980s and 1990s many avenues were explored in contrast agent research, among them iron chelates analogous to the gadolinium chelates, e.g. Fe-DTPA and Fe-tCDTA. These compounds are positive enhancers that were thought to be usable for indications similar to the gadolinium-based ECF-space agents.
However, the relaxivities of the Fe-chelates were substantially weaker; sufficient enhancement required a major dose increase. Moreover, toxicological aspects added to the abortion of the development of iron chelates; severe acute side effects were observed, and long-term effects were predicted [⇒ Wesby 1984, ⇒ Tweedle 1988, ⇒ Duewell 1991 ⇒ Tweedle 2018].
Iron oxides. Iron-oxide particles are incorporated into cells of the reticulo-endothelial system (RES) through phagocytosis. This opens a selective access route to liver, spleen, lymph nodes, and bone marrow. The particles can also be applied for receptor and antibody imaging, as well as perfusion imaging of the heart and the brain.
The agents can either be positive (T1) or negative (T2/T2*) enhancers, depending on particle size, composition and concentration, saturation magnetization of the material, as well as equipment hardware and pulse sequences used. Three classes of superparamagnetic nanoparticles exist; they depend on their particle size (Table 13-04). Their biodistribution is determined by size, shape, charge, hydrophilicity, chemical composition, and surface coating (cf. negative contrast agents | susceptibility effects) [review article: ⇒ Corot, ⇒ Laurent 2008, ⇒ Dallet 2021].
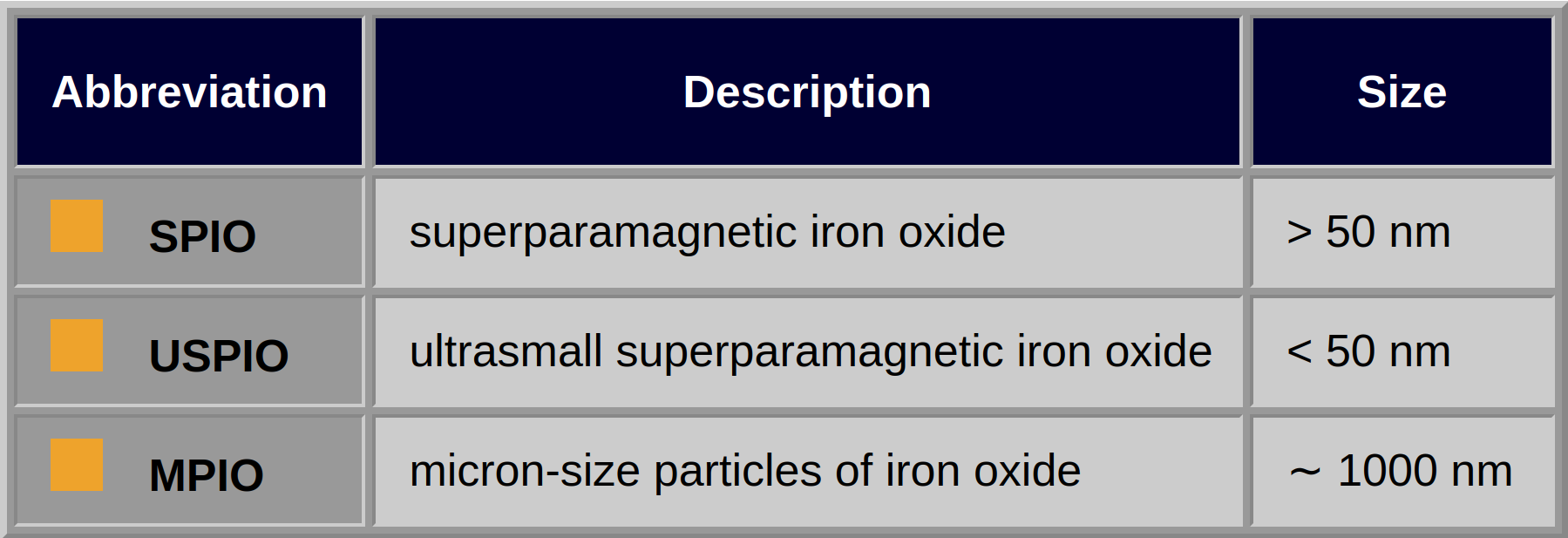
Table 13-04:
Classes of superparamagnetic nanoparticles depending on their particle size.
The majority of compounds are polydisperse (more than one size population of iron oxide crystals) and polycrystalline (particle aggregates). Actively targeted iron oxides preferably contain smaller superparamagnetic labels [⇒ Chertok 2008]; they are monodisperse (only one size population of iron oxides) and monocrystalline (= MION, i.e., each particle consists of only one crystal).
Used intravenously, such particles should possess a (postulated) particle size smaller than 50 nm in diameter so that they are not entrapped during their passage through the lungs.
Because of severe side effects, among them serious and life-threatening anaphylactic reactions and immune responses, some iron oxide nanoparticles were withdrawn from most markets. Iron can also be deposited in the choroid plexus.
For some time, ferumoxtran-10 is being clinically re-evaluated. It is claimed to detect early-stage cancer metastases in lymph nodes in patients with progressive prostate cancer [⇒ Frantellizzi 2020, ⇒ Harisinghani 2003]. Similarly, ferumoxytol has been proposed as a vascular contrast agent, particularly for patients with chronic kidney disease [⇒ Weissleder 1990, ⇒ Stoumpos 2019]. It was originally designed as an intravascular contrast agent but commercialised as an intravenous iron therapy for anemia.
The liver has been selected as the primary organ for developing passive targeting compounds: vascular, hepatobiliary, and reticuloendothelial. Different possible enhancement patterns are depicted in Figure 13-12.
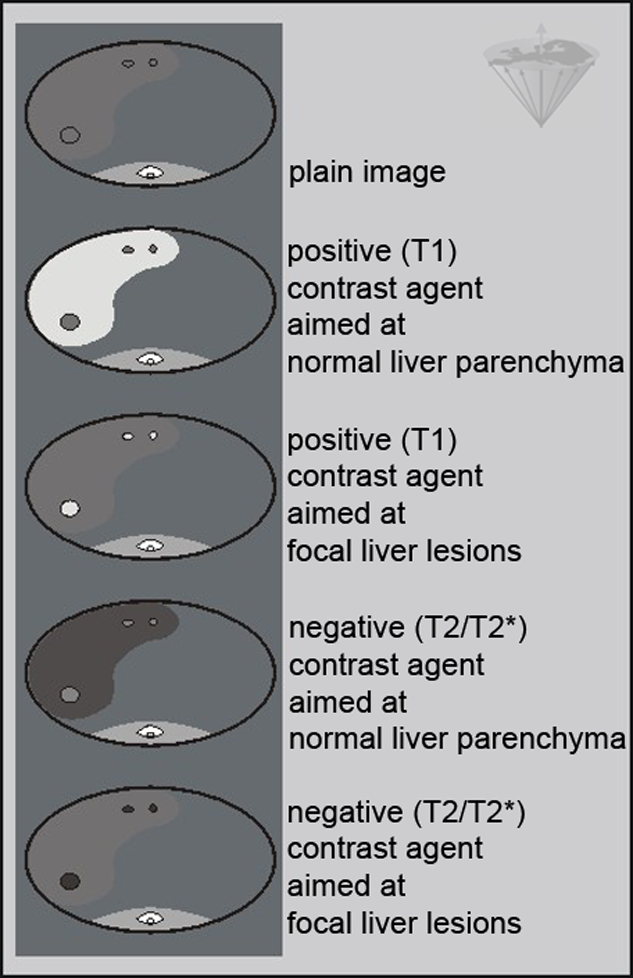
Figure 13-12:
Enhancement patterns of different liver contrast agents (modified from ⇒ Leander).
Aside from the vascular structures, either the hepatocytes or the RES can be targeted. Vascular structures and highly vascularized lesions are commonly highlighted by dynamic examinations with the conventional low molecular weight contrast agents (Figure 13-13). Dynamic imaging is discussed in detail in Chapter 16.
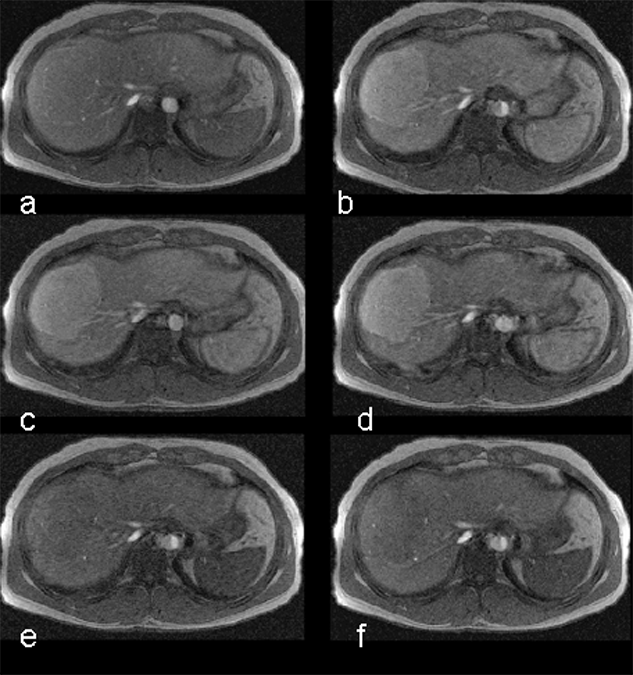
Figure 13-13:
Focal nodular hyperplasia (FNH) of the liver. Dynamic enhancement with Gd-DOTA; first highlighting of the arterial vessels, then strong enhancement of the tumor during the early phase of arterial enhancement; followed by the depiction of the veins. Timescale approximately 90 seconds.
Gd-EOB-DTPA and Gd-BOPTA are two positive gadolinium based agents with lipophilic side groups. Gd-EOB-DTPA [⇒ Runge] is a targeted liver agent, whereas Gd-BOPTA is a multipurpose contrast agent, well suited for liver imaging [⇒ Spinazzi].
Although the chemical composition of Gd-BOPTA appears similar to that of the extracellular gadolinium agents, it combines both extracellular and liver-targeted properties, because some 5% of it is excreted through the liver, as is shown in this case of multiple metastases (Figure 13-14).
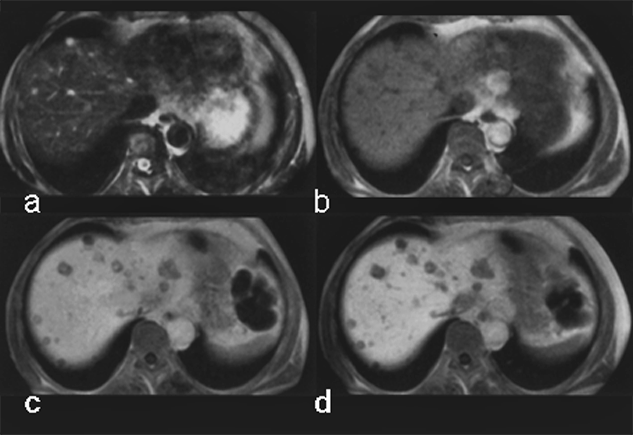
Figure 13-14:
Gadolinium-BOPTA in liver metastases of a pancreatic tumor.
(a) plain T1-weighted GRE sequence; (b) plain T2-weighted GRE sequence; (c) enhanced T1-weighted GRE sequence 40 minutes after injection; (d) T1-weighted GRE sequence 90 minutes after injection.
Ferumoxides, or super-paramagnetic iron oxides, are negative enhancers taken up by the normal liver, which contains reticuloendothelial cells, but not by lesions that lack reticuloendothelial cells (Figure 13-15) [⇒ Paley; cf. iron oxides].
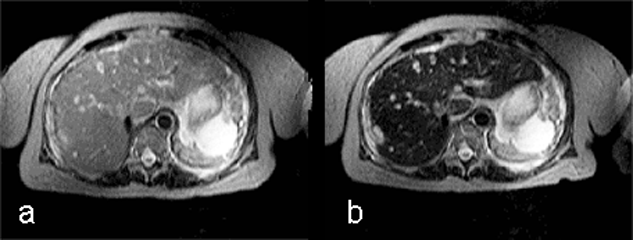
Figure 13-15:
Example of a negative liver contrast agent (ferumoxide). This particulate agent is taken up by endothelial and Kupffer's cells. They darken the liver tissue due to their effective shortening of the T2 relaxation time.
T2-weighted GRE: (a) precontrast; (b) postcontrast. The liver metastases are well delineated on the post-contrast image; with this agent, the normal liver tissue becomes black.
Manganese was the first element applied to enhance pathologies in MR imaging; its use was described by Lauterbur, Mendonça-Dias and Rudin in 1978 [⇒ Lauterbur 1978]. They imaged five dogs with myocardial infarctions after injecting a manganese salt solution and were able to highlight the lesions. Manganese was chosen because it is only moderately toxic and its distribution and elimination had been investigated earlier.
Yet, gadolinium became the element of choice for MR contrast agents because of its high relaxivity and patent issues. However, it is an element foreign to the human body whereas manganese is an endogenous metal and essential trace element. During the last 25 years, there is increasing activity in the preclinical research of Mn-based agents (MEMRI — manganese-enhanced magnetic resonance imaging). In addition to it, there are numerous other proposals for manganese-based agents. Most of them are stuck in the pre-clinical phase of development, mostly trying to establish diagnostic efficacy and general short-term and long-term safety [⇒ Henoumont 2023].
However, the only compound available on the market for some time was Mn-DPDP (mangafodipir — Teslascan), mainly used for liver imaging; it was withdrawn from US and EU markets in 2012 because of unsuccessful marketing, the necessity of slow infusion, and concerns over potential toxicity. While on the market for enhance liver imaging, mangafodipir was the preferred agent in patients with kidney problems. It remains to be seen whether this compound will return to the market for cardiac or other applications.
Mn-DPDP (Figure 13-16) is a positive multipurpose agent taken up by, e.g., the hepatocytes [⇒ Rummeny 1997]. The contrast enhancement seems to be connected to a limited release of manganese ions. The enhancement is long lasting.
Oral or intravenous MnCl2 can have similar effects. A number of oral manganese drugs were proposed and introduced but their absorption in the human body is not exactly calculable and predictable.
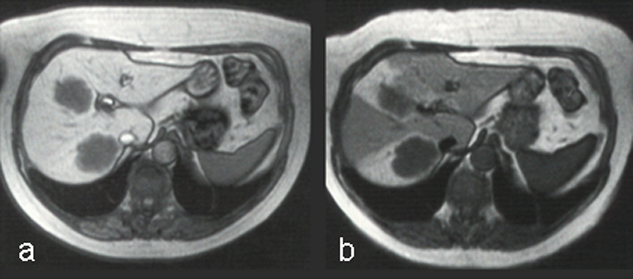
Figure 13-16:
The uptake of Mn-DPDP (mangafodipir) in the liver relies on the ability of hepatocytes to excrete metal ions. Manganese separates from the DPDP-complex and is taken up by the hepatocytes.
T1-weighted GRE images. (a) The metastases are well delineated 15 minutes after the injection, and (b) even 24 hours after administration some of the contrast agent remains.
Manganese also has an affinity for the myocardium and could act as biomarker in heart disease. Manganese ions compete with calcium for entry into cardiac cells. There the ions bind to macromolecules and influence the relaxation of cell and tissue water. Heart diseases gradually inactivate calcium transport mechanisms due to lower metabolic activity. Thus, manganese uptake is reduced accordingly; manganese-induced changes of tissue relaxation reflect tissue calcium homeostasis and thus myocardial viability (Figures 13-17 and 13-18) [⇒ Skjold 2004, ⇒ Skjold 2007] and allows the assessment of stunned and viable myocardium [⇒ Spath 2021, ⇒ Singh 2023].
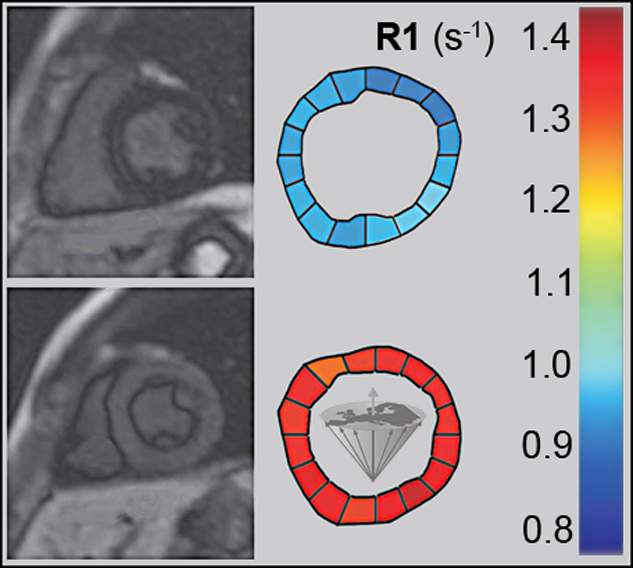
Figure 13-17:
Mn-DPDP: A short axis single shot IR Turbo-FLASH image of a heart before contrast agent application (top), and another image of the same heart with the same parameter settings one hour after the end of the contrast agent infusion (bottom).
To the right of each image sectoral divisions of the left ventricle are shown, depicting R1 color maps of the respective slice. The left ventricle shows a marked change in contrast (modified from Skjold 2004).
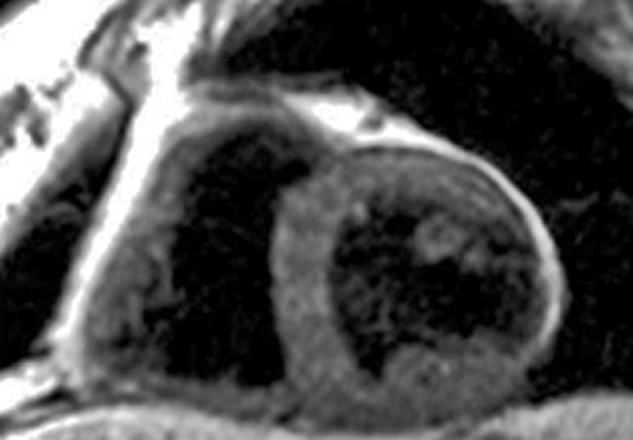
Figure 13-18:
Follow-up of a cardiac infarction. Manganese-enhanced myocardium (Mn-DPDP, mangafodipir) showing an infarcted region in the lateral wall of the left ventricle (dark wall region).
(Image courtesy of Dr. Arne Skjold, Trondheim, Norway).
In addition to imaging of the liver and the heart, manganese-enhanced MRI with mangafodipir has a wide range of potential applications. Research is focused upon both depiction of brain damage and functional mapping of neural pathways to map brain activation independently and with higher contrast than measurements of hemodynamics in fMRI [⇒ Sudarshana 2019].
During the development of mangafodipir it was discovered that it and its metabolite manganese pyridoxyl ethyl-diamine (Mn-PLED) possess therapeutic properties. Mn-DPDP’s contrast enhancement in MR imaging relies on the release of manganese from the chelate, the therapeutic activity depends on manganese that remains bound to the chelate [⇒ Jynge 2020]. Mangafodipir may have a cardioprotective role in reducing reperfusion injury [⇒ El‐Saadi 2023].