






 espite the fact that inherent contrast in MR imaging can be manipulated to a much greater extent than in other imaging techniques, certain diagnostic questions cannot be answered easily and require the application of contrast agents (Figure 13-01).
espite the fact that inherent contrast in MR imaging can be manipulated to a much greater extent than in other imaging techniques, certain diagnostic questions cannot be answered easily and require the application of contrast agents (Figure 13-01).

Figure 13-01:
Nature likes mimicry; radiologists like to highlight lesions. With plain photography (or MR imaging), the object of the examination might be visible but nor clearly delineated. Changing contrast with an extrinsic agent may help … for instance, painting the wall in the background or injecting a contrast agent.
In general, contrast manipulation in MR imaging by application of contrast agents (sometimes also called molecular imaging) is most useful when inherent contrast cannot be attained successfully.
Since it is nearly impossible to alter the water content of tissues, contrast agents on the market or in clinical or pre-clinical trials focus on relaxation time and susceptibility changes.
As early as 1946, in one of the first papers describing NMR, paramagnetic catalysts were mentioned to accelerate the T1 relaxation process [⇒ Bloch]. This concept was recognized by Paul C. Lauterbur shortly after his invention of MR imaging and tested and proved in imaging studies in animals: the first pathologies enhanced by a contrast agent [⇒ Lauterbur].
The most efficient elements are listed in Table 13-01. Particulate agents form a different class. Important goals and requirements for the development and use of MR contrast agents are listed in Table 13-02.
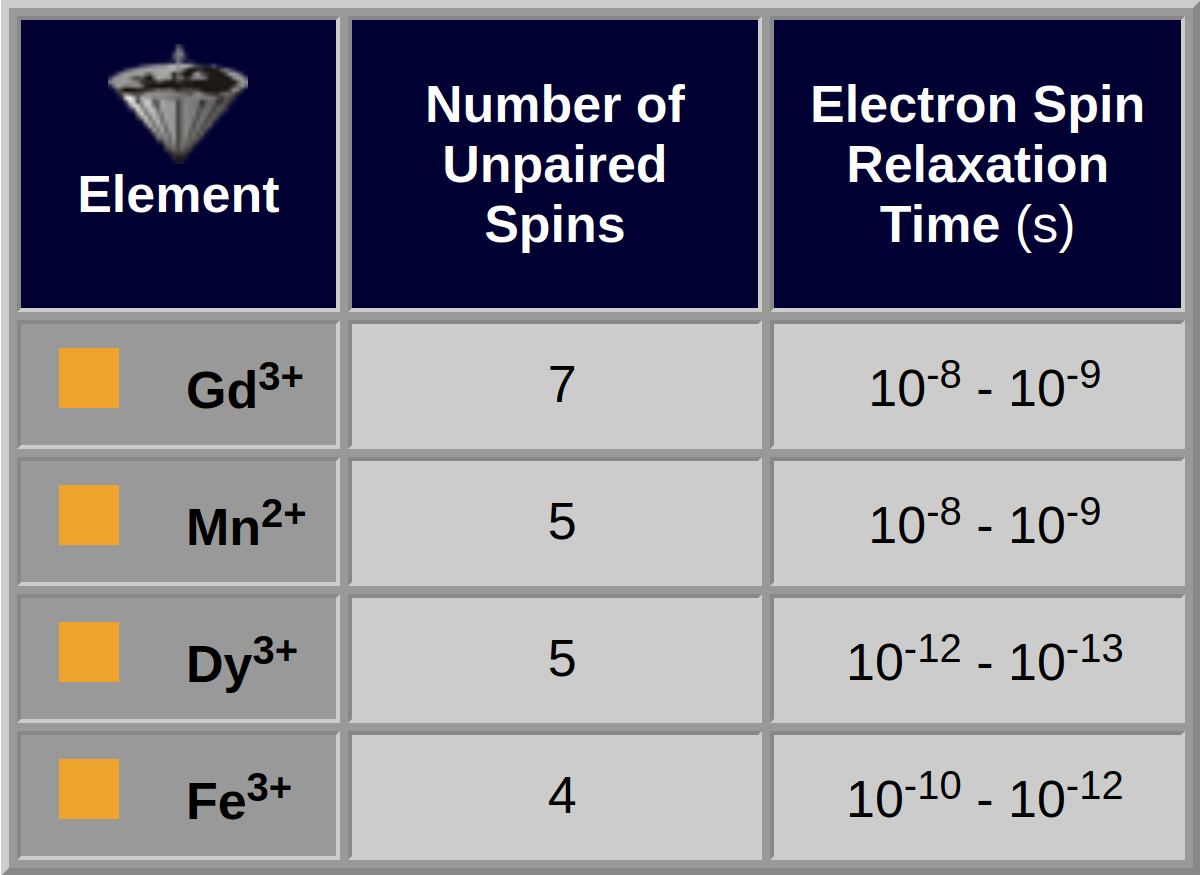
Table 13-01:
Some paramagnetic elements and their properties: gadolinium, manganese, dysprosium, and iron.

Table 13-02:
Primary and secondary goals and requirements for the development of contrast agents for magnetic resonance imaging.
In many instances, the pattern of enhancement of paramagnetic contrast agents in MR imaging is very similar to that of contrast-enhanced x-ray CT. However, it should be taken into account that in reality MR contrast agents behave differently from CT agents and do not in any case follow the CT enhancement patterns: In x-ray CT one sees the agent, in MR imaging one sees the effect of the agent on tissues.
Many efforts in contrast agent development were channeled in certain directions more by the relative ease of chemical synthesis than by the goal of specific medical applications or product safety. Thus, when applied properly, contrast agents available for clinical routine examinations today are safe and good enhancers — however, they are unspecific. This means that they do not highlight specific pathologies but rather unspecific pathological tissue changes.
A detailed and exhaustive description of the state of R&D in the field can be found in a review from 2019 [⇒ Wahsner 2019].
At present, paramagnetic contrast agents are the most frequently used. Yet, despite the fact that approximately one thousand compounds were developed during the last forty years, only very few agents are on the market. However, the number of trade names is far higher and an overview of different brands of the same product can be difficult.
Table 13-03 gives an overview of some of the MR contrast agents currently in use, already withdrawn from the market, or being developed.
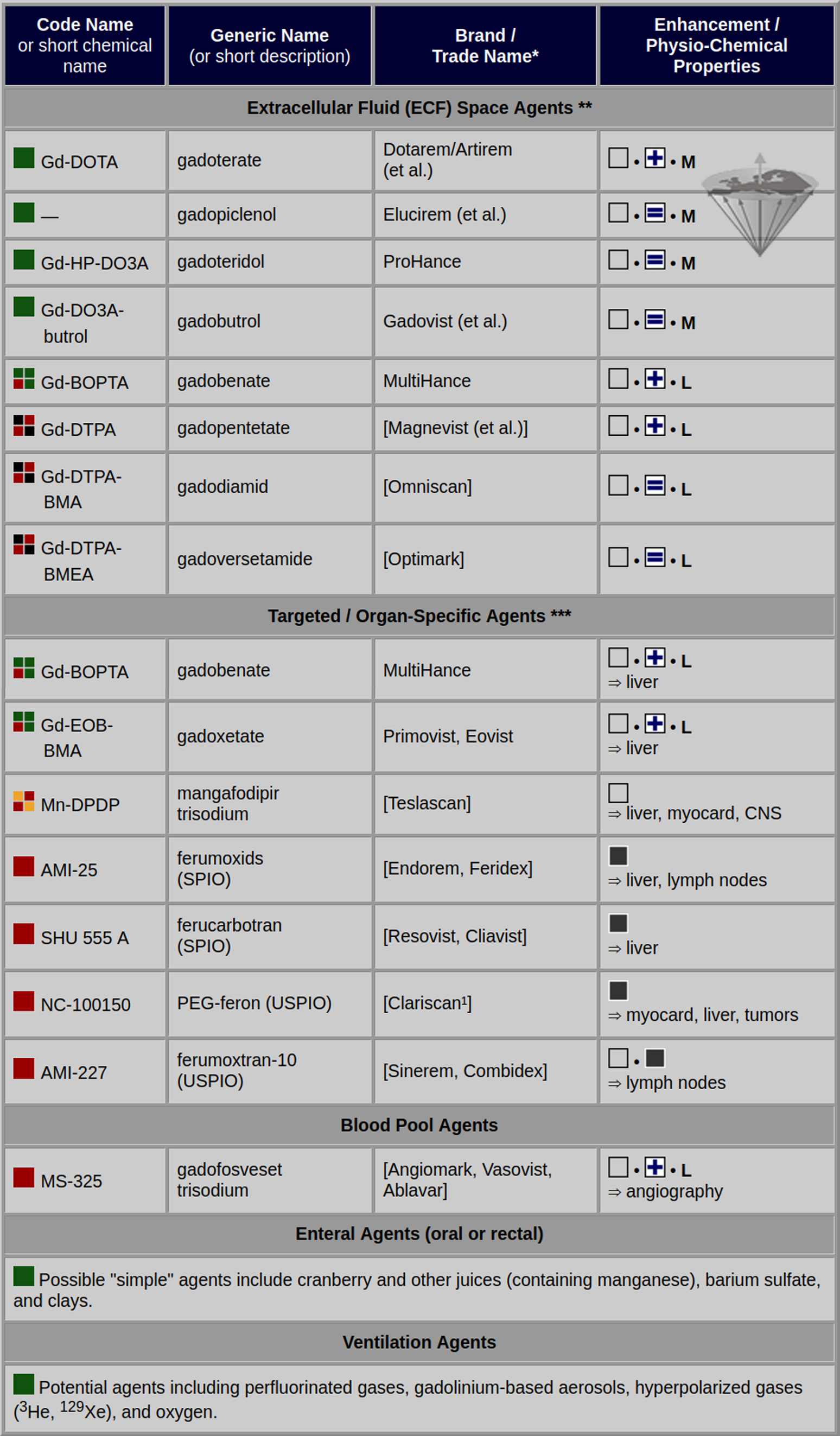
Table 13-03:
Classification of some magnetic resonance contrast agents being developed, to be approved, approved, marketed, or withdrawn from development or the market. There are numerous other agents in development.
![]() = agent available for clinical and/or research application;
= agent available for clinical and/or research application;
![]() = agent available for clinical and/or research application with limited indications;
= agent available for clinical and/or research application with limited indications;
![]() = development interrupted or discontinued;
= development interrupted or discontinued;
![]() = agent withdrawn from one or all major markets;
= agent withdrawn from one or all major markets;
![]() = agent suspended by the European Medicines Agency (10 July 2017).
= agent suspended by the European Medicines Agency (10 July 2017).
![]() = positively enhancing agent;
= positively enhancing agent; ![]() = negatively enhancing agent.
= negatively enhancing agent.
![]() = globally charged agent;
= globally charged agent; ![]() = globally neutral agent.
= globally neutral agent.
M = macrocyclic; L = linear.
¹ Do not mistake for the generic Gd-DOTA agent of the same name.
* = trademark or registered trademark;
** = with high concentrations, negative contrast can be achieved (e.g., first-track bolus);
*** = all ECF agents are also kidney-specific agents;
(et al.) = the compund is sold under different trade names.