





![]() n traditional chemical/analytical NMR spectroscopy, the sample is placed inside the coil and the signal from the whole sample is observed.
n traditional chemical/analytical NMR spectroscopy, the sample is placed inside the coil and the signal from the whole sample is observed.
This is fine as long as we have a homogeneous sample. However, if we want to obtain signals from living animals or humans, this approach would lead to signals from a mixture of the different tissues within the detecting region of the coil.
Therefore, to obtain in vivo spectra from a particular type of tissue, it is necessary to limit or localize the volume from which we actually detect the signal.
The simplest localization technique is to use a surface coil. The size of the volume from which the signal is received is determined by the size and configuration of this coil. Whereas this technique is very simple and gives a good signal-to-noise ratio, it has a number of disadvantages, including contamination from surface tissue, a decrease in signal from tissues at increasing depth, and large variations of the flip angle across the excited volume. The latter problem can be partly reduced if a standard RF coil is used for exciting the whole sample and a surface coil is used to detect the signal from the localized volume, or if specialized (adiabatic) RF pulses are applied.
The other techniques discussed here allow a volume to be defined on previously acquired positioning images, thus reducing the problem of contamination from other tissues.
The quality of the magnetic field within the volume of interest is optimized by means of localized shimming. Smaller volumes allow for better shimming, but as the signal in the spectrum is proportional to the volume size, very small volumes are impractical except for ¹H spectroscopy.
For localized spectroscopy of nuclei other than ¹H we must be able to obtain a localized proton signal from the same volume; this is required for localized shimming since the sensitivity of the other nuclei is too poor.
A number of localization schemes have been proposed. Four of the most widely used schemes will be discussed here. An extended discussion of spectroscopy techniques can be found in the papers by Matson and Weiner as well as Sauter and collaborators [⇒ Matson 1999, ⇒ Sauter 1992].
The stimulated echo is one of five echoes generated by a three 90° pulse RF sequence. If these three RF pulses are made selective in orthogonal planes a stimulated echo will only be obtained from the volume located at the intersection of the three planes.
The signal is attenuated by T1 decay between the second and third pulse and by T2 decay in the other two intervals. The problem of T2 decay makes the scheme best suited to ¹H rather than ³¹P spectroscopy, due to the short T2 values of phosphorus.
A number of acronyms are used to describe pulse sequences of this type, including STEAM [⇒ Frahm 1988], STEV [⇒ Kimmich 1987], and VOSY (Figure 05-07).
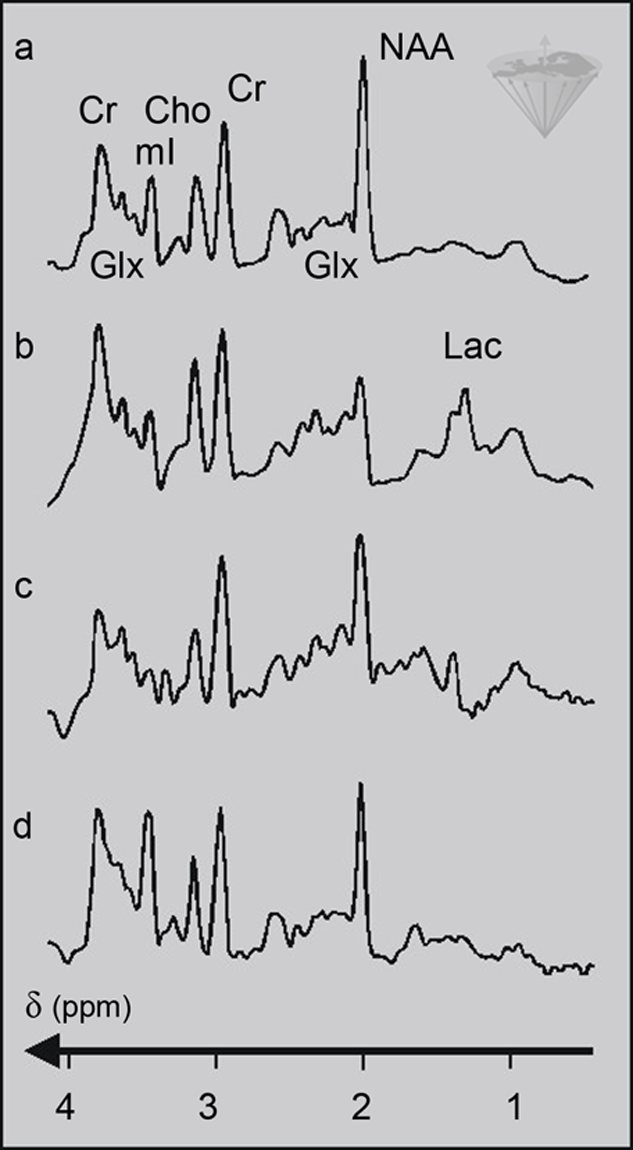
Figure 05-07:
STEAM proton spectra of (a) a normal human brain; (b) hypoxia; (c) hepatic encephalopathy; (d) Alzheimer’s disease. The abbreviations are explained in Table 05-01.
The point resolved spectroscopy localization technique (PRESS) consists of a spin-echo train with two 180° pulses. Each pulse is applied in the presence of an orthogonal gradient which allows the selection of a volume element within the sample to be examined. PRESS needs relatively long echo times.
The ISIS (Image Selected In Vivo Spectroscopy) sequence consists of eight steps [⇒ Ordidge 1986]. In each step, a sequence of selective inversion pulses is applied, followed by a nonselective excitation pulse.
The combination of inversion pulses and receiver phases is unique for each of the steps. By combining the resulting eight signals in the correct manner, the signals from the volume of interest will add up, while those from the rest of the sample will cancel each other.
Since the FID rather than an echo is observed, there is no T2 decay. This makes the sequence well-suited to ³¹P spectroscopy.
For ¹H spectroscopy, the sequence should be modified so that the very large water signal from the outer volume (i.e., the rest of the sample) is suppressed to avoid dynamic range problems.
This is done in an OSIRIS sequence, a modified form of the ISIS sequence in which the signal from the outer volume is suppressed with a noise pulse. Because of the eight steps involved, the sequence is rather susceptible to motion artifacts, since any movement will lead to incomplete cancellation in the outer volume.
For the ISIS scheme to work efficiently, the magnetization must come close to full recovery before the next pulse is applied, so rather long TR values are required.