





 he composition of tissues in the human body changes with age. This is of particular importance in the brain, where the water content decreases and the myelin content increases dramatically during the first years of infancy.
he composition of tissues in the human body changes with age. This is of particular importance in the brain, where the water content decreases and the myelin content increases dramatically during the first years of infancy.
Consequently, T1 and T2 relaxation times of brain tissue decrease. At birth, the infant brain consists of 93-95% of water and has long T1 and T2 relaxation times (Figure 09-12). There is a fast fall in water content to 82-84% during the first two years of life as myelination takes place.

Figure 09-12:
Brain images of (a) an infant of 11 months, and (b) an adult at 0.5 T. The same pulse parameters were used (SE: TR = 500 ms, TE = 20 ms). Windowing is slightly different.
Still, image contrast, in particular contrast between gray and white matter is obviously not the same because in the infant myelination has not reached the adult stage and both T1 and T2 of white matter are higher than T1 and T2 of gray matter.
Therefore, it is necessary to adjust the timing parameters of all pulse sequences accordingly. When using IR sequences at mid-field (0.5 T) in the neonatal period, a TR of 3000 ms and TI of 1000 ms are required to produce images with useful soft tissue contrast. The TR and TI can be halved by the time the child is two years of age. When using SE sequences, TR has to be prolonged accordingly for T2-weighted images. The use of the same pulse parameters in infants as in adults will lead to images without diagnostic value (Figure 09-13) [⇒ Holland 1986]. In children aged three to six years, the sequence parameters of adults can be used.
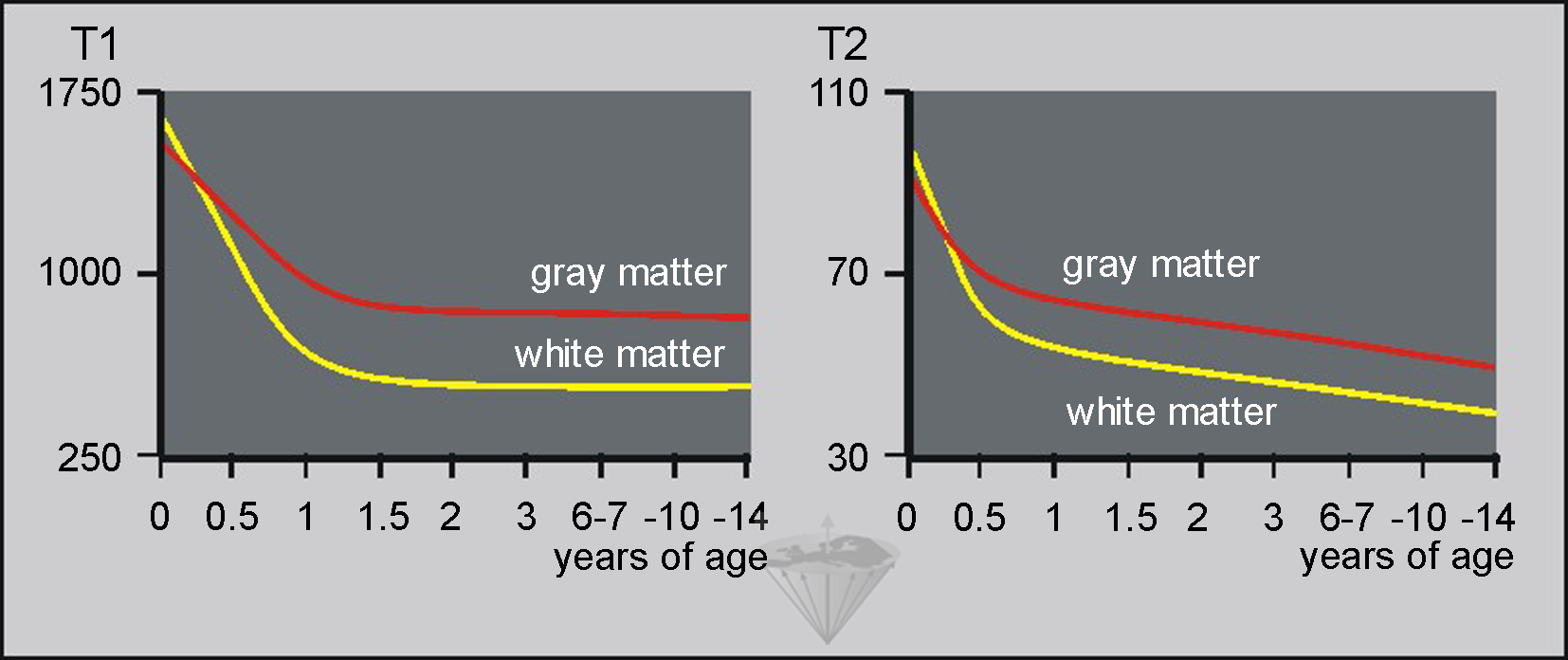
Figure 09-13:
(Left) T1 relaxation times and (right) T2 relaxation times of gray and white matter by age in milliseconds. Note that from birth until approximately six months of age, both T1 and T2 of gray matter are shorter than T1 and T2 of white matter. In vivo measurements at low field; standard deviation approximately 25%.