






 agnetic resonance contrast agents aim at changing signal intensity and thus image contrast. The main contrast parameters in MR imaging are proton density, the relaxation times, and magnetic susceptibility.
agnetic resonance contrast agents aim at changing signal intensity and thus image contrast. The main contrast parameters in MR imaging are proton density, the relaxation times, and magnetic susceptibility.
It is rather difficult to alter the water content of tissues. Therefore, the magnetic properties have been the major target for the development of contrast changing agents. Magnetic susceptibility describes the ability of a material or substance to become magnetized by an external magnetic field.
So, let’s change — for instance — the field lines (Figure 12-01).

Figure 12-01:
In which direction do we go? Where are the field lines?
All substances are diamagnetic. A strong external magnetic field speeds up or slows down the electrons orbiting in atoms in such a way as to oppose the action of the external field. These materials partly expel from their interior the magnet field in which they are placed.
Certain materials have ferromagnetic properties, among them iron, nickel, cobalt, and their alloys. Ferromagnetic materials are strongly attracted by magnets. In ferromagnetic materials, there is a strong coupling of the individual magnets, resulting in their lining up parallel to one another (Figure 12-02a). They lose their magnetic properties when heated above a temperature known as the ‘Curie point’ (770° C for iron, 358° C for nickel, 1120° C for cobalt).
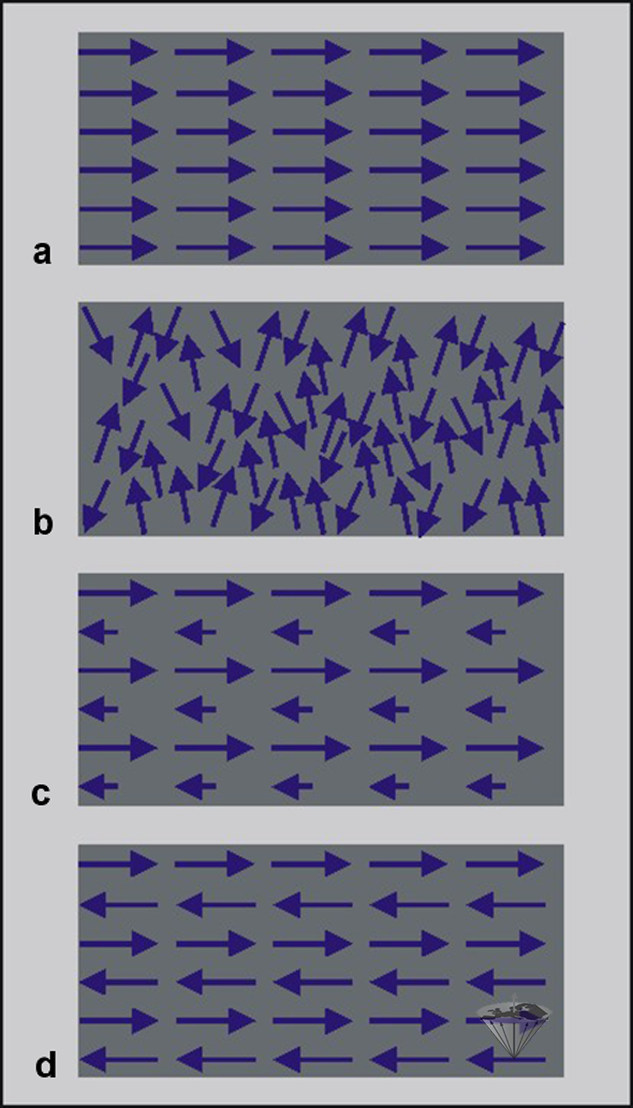
Figure 12-02:
(a) ferromagnetic material: atomic magnets are strongly coupled;
(b) paramagnetic material in an external field: atomic magnets are weakly coupled;
(c) ferrimagnetic material: weak overall magnetism;
(d) antiferromagnetic material: atomic magnets coupled in an antiparallel manner, resulting in no magnetism.
Then they begin to show a kind of magnetic behavior which as called paramagnetic. Many other elements and compounds are paramagnetic at all temperatures, among them oxygen, gadolinium, and manganese (see also Chapter 4). Paramagnetism is due to the presence of little colonies of atomic magnets, in which the individual magnets are weakly, if at all, bound to one another and therefore capable only of random orientation in the absence of an external field (Figure 12-02b). Paramagnetic substances are feeble in their response to an external magnetic field.
Superparamagnetic substances have a substantially higher susceptibility.
Ferrimagnetic materials, such as ferrites, are also coupled in an antiparallel fashion, but the overall effect of the individual magnets pointing in one direction exceeds. Thus, the net effect is that of weak overall magnetism (Figure 12-02c).
Antiferromagnetic materials consist of elementary magnets coupled together in opposite directions, resulting in zero net magnetization (Figure 12-02d).
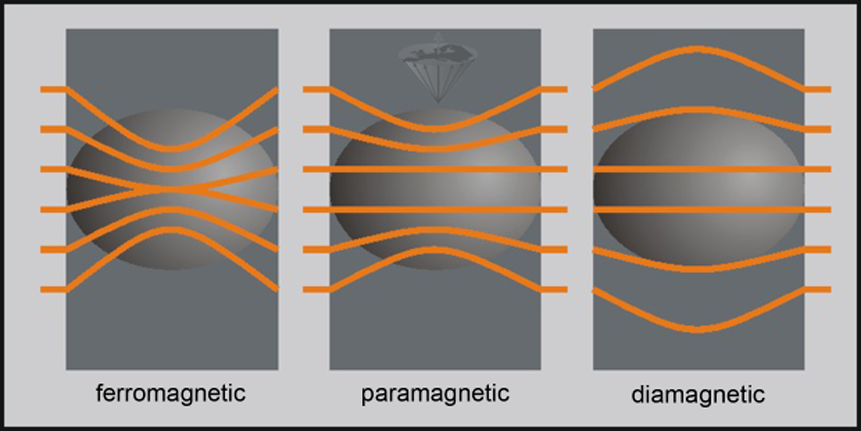
Figure 12-03:
Different magnet compounds and outside magnetic fields.
Diamagnetic substances possess a negative susceptibility: χ ≤ 0, paramagnetic substances a small positive susceptibility: χ > 0, superparamagnetic substances a susceptibility 2-3 orders of magnitude stronger than paramagnetic substances: χ > 0, and ferromagnetic substances a large positive susceptibility: χ >> 0.
For diamagnetic material, the value of susceptibility is always negative; for paramagnetic, superparamagnetic, and ferromagnetic substances positive. Figure 12-03 shows the response of diamagnetic, paramagnetic, and ferromagnetic substances to an outside magnetic field.
To be able to develop and compare contrast agents there has to be agreement of how their properties should be measured. Table 12-01 summarizes the latest version (1997) of the Standard Nomenclature of Magnetic Resonance Contrast Agent Terms for relaxation times, relaxation rates, and relaxivities [⇒ EMRF 1997].
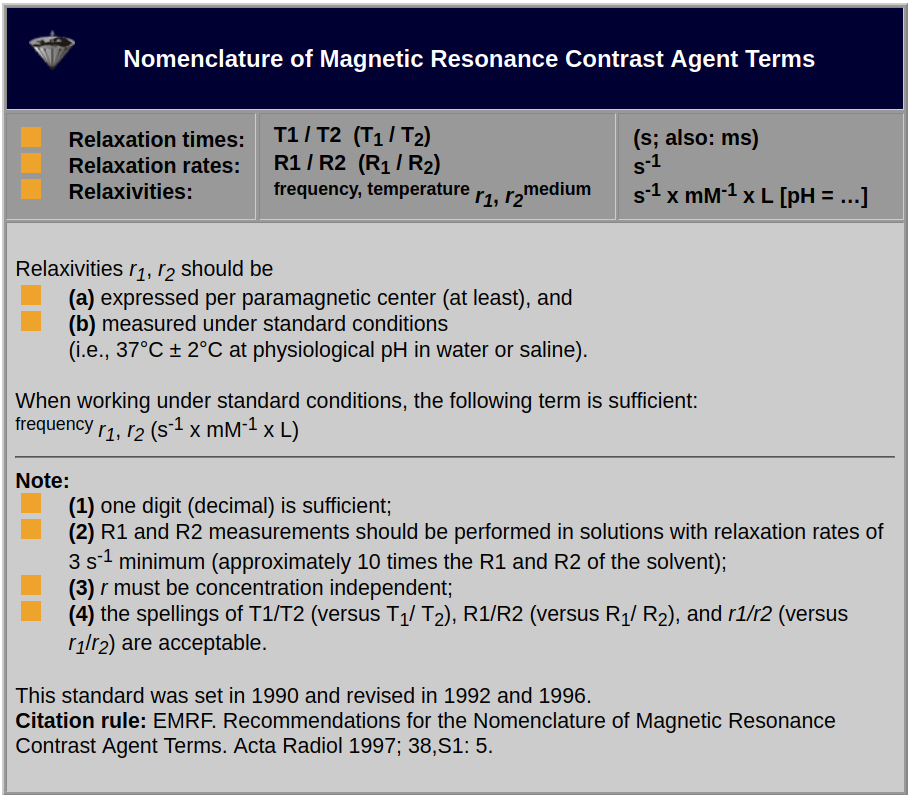
Table 12-01:
Recommendations for the Nomenclature of Magnetic Resonance Contrast Agent Terms.
Terminology and Understanding the Fundamentals. Some physicians and researchers are using ‘apparent’ T1 for cardiac mapping and mix T2* (in reality T2app), R2* and r2* randomly. In some publications, additional terms are also used wrongly or confusingly, for instance T1* (a term which is not appropriate because T1 is not affected by susceptibility effects) for an apparent T1 (T1app or T1influx).